Introduction
Ticks are widespread haematophagous
ectoparasites that infest several vertebrate groups, including reptiles. In
principle, ticks are deleterious to their vertebrate hosts because they consume
resources from them and, therefore, may result in a decrease in host body
condition (Smyth et al. 2014; Megía-Palma et
al. 2020c), and even cause anaemia in severe
infestation cases. Hosts infested with ticks may suffer from low haemoglobin
and haematocrit levels (Pfaffl et
al. 2009; Lanser et al. 2021), but some reptile
species can maintain their carrying oxygen capacity (Bull and Burzacott 1993; Knapp et al. 2019) and haemoglobin levels (Albuquerque et al. 2023) regardless of
their tick infestation levels. Besides their direct effects on hosts, ticks can
also transmit a variety of pathogens to reptiles, such as bacteria (e.g. Borrelia
spp., Rickettsia spp.; Mendoza-Roldan et al. 2021) and viruses
(e.g. Crimean-Congo haemorrhagic fever virus; Kar et al. 2020), but the ability of
those microorganisms to cause clinical symptoms on reptile hosts is
understudied.
The tick Ixodes ricinus is
a generalist hard tick belonging to the Ixodidae family. As in all ixodid
ticks, the life cycle of this species consists of three stages: larva, nymph,
and adult. The immature stages (larvae and nymphs) infest small mammals, birds
and lizards, especially during late winter and spring in Europe (Norte et al. 2012; Kahl and Gray 2023), while in North Africa they can be found until mid-summer (Dsouli et al. 2006; Soualah-Alila
et al. 2015). The adult, by contrast, infests large
hosts such as carnivores and ungulates. All stages feed on the host’s blood
once, and each stage may be attached to the host body for up to several weeks.
Immature stages detach from the host body to moult, while the adult female
detaches into the vegetation or leaf litter to lay their eggs (Kahl and Gray 2023). Ixodes ricinus is the main vector of Borrelia
burgdorferi s.l. in Europe, and also of the genospecies Borrelia
lusitaniae, associated with clinical symptoms in humans (an accidental
host; Lopes de Carvalho
et al. 2008). The lizard Psammodromus algirus acts
as a competent reservoir of Borrelia spp. in Mediterranean areas of
Western Europe and North Africa, which is transmitted by this vector tick (Dsouli et al. 2006). Local prevalence of immature I. ricinus in this lizard
species can reach nearly 90%, with a peak infestation intensity of 16 ticks per
host in Portugal (Norte et al.
2015) and up to 58 in Spain (Carbayo et al. 2019). However, the infestation of P. algirus with hard ticks is
rare and rather local. In P. algirus, tick infestation is also expected
to facilitate other parasite infections by increasing the susceptibility to
infestation by other arthropod vectors (the co-infection facilitation
hypothesis; Rodgers and
Bolnick 2024). For example, tick infestation could
facilitate the infestation by some haematophagous mites (but also see Ferreira et al. 2023), which are common ectoparasites of P. algirus (Álvarez-Ruiz et al. 2018; Drechsler et al.
2021), and some of them can transmit blood
parasites (Haklová-Kočíková
et al. 2014; Megía-Palma et al. 2023). Specifically,
the transmission of the blood parasite genus Karyolysus to lizards is
thought to involve mainly haematophagous mites (genus Ophionyssus)
containing infectious sporozoites, while blood parasites of the genus Hepatozoon
may be transmitted by a wide variety of invertebrates, including hard ticks (Telford 2008).
This could further contribute to an impaired overall health of the hosts (Megía-Palma et al. 2022). This association is expected given that tick-infested P.
algirus lizards with a decreased immune system (Veiga et al. 1998) and those with low body condition (Amo et al. 2007) are more
susceptible to blood parasite infection (Ferreira et al. 2023), but in other
lizards, this depends on the environmental context (Wu et al. 2019).
Therefore, tick infestation is expected to
impact the lizards’ body condition, breeding success, and fitness (Megía-Palma et al. 2018; 2020c). Since the highest I. ricinus pressure concurs in spring
with the lizard’s reproduction peak, energetic trade-offs may arise in lizard
hosts that may allocate resources either to reproduction (e.g. production of
sexual traits, defence of territories or generation of high-quality offspring)
or to counteract parasite pressure (e.g. raising the immune activity or
recovering from blood loss to maintain blood homeostasis and oxygen carrying
capacity). Moreover, the costs derived from such trade-offs may differ for
males and females because reproduction energetic demands may be higher in males
during spring, whereas females have a higher reproductive investment later in
summer (Arakelyan et al. 2019; Megía-Palma et
al. 2024a). For example, as a consequence of
producing more testosterone, P. algirus males may decrease their immune
defences, which may lead to an increase in tick load (Salvador et al. 1996; Veiga et al. 1998). This makes male lizards more susceptible to tick infestations
than females during spring (Václav
et al. 2007; Dudek et al. 2016). Ultimately, this
could negatively impact sexual selection during the mating season, since
females of P. algirus may prefer healthier, non-infested males (Martín et al. 2007; Comas et al. 2025).
Hernández-Rojas
et al. (2025) have recently reported on the
geographic distribution of I. ricinus infesting several species of
lacertid lizards across the Iberian Peninsula. Here, we analysed the geographic
variation of tick prevalence and intensity in P. algirus, and the
relationships among the cellular component of its immune system, blood
physiology, and tick intensity. To this end, we captured P. algirus
lizards across its geographic distribution on the West, Centre, and South of
the Iberian Peninsula, and North-western Africa. We examined the geographic
variation in prevalence and intensity, and across host life stages (juveniles
versus adults) and sexes. We also analysed the relationship between tick
intensity and leucocyte profiles and total haemoglobin concentration. We expect
that lizard males will be more frequently infested with ticks than females (Václav et al. 2007; Dudek et al. 2016), and adult lizards will carry more ticks than juveniles (Dudek et al. 2016). Moreover, and in line with the co-infection facilitation
hypothesis (Rodgers and
Bolnick 2024), which predicts that infested lizards
could be more susceptible to infections by other parasites, we evaluated
whether there was a significantly positive relationship between tick
infestation and blood parasite infection.
Material and Methods
Field sampling and general
procedures
Lizards were
captured from March to July 2022 by hand or using a noose (García-Muñoz and Sillero 2010) in different sites in Portugal, Spain, and Morocco (Fig. 1).
Lizards from the Centre of Spain (Madrid) were captured in 2023. Based on the
geographic proximity and sample size of some of the sites (Fig. 1), we
used the following groups for the subsequent statistical analyses: Mafra, Águas
de Moura, Grândola, Granada, Huelva, Madrid, North Morocco, and South Morocco (Fig. 1). In
Huelva, an experiment was conducted which involved the inoculation of a
subsample of males with an endotoxin (see Comas et al. 2025). Hence, in this
study, we included only control males and females from Huelva.
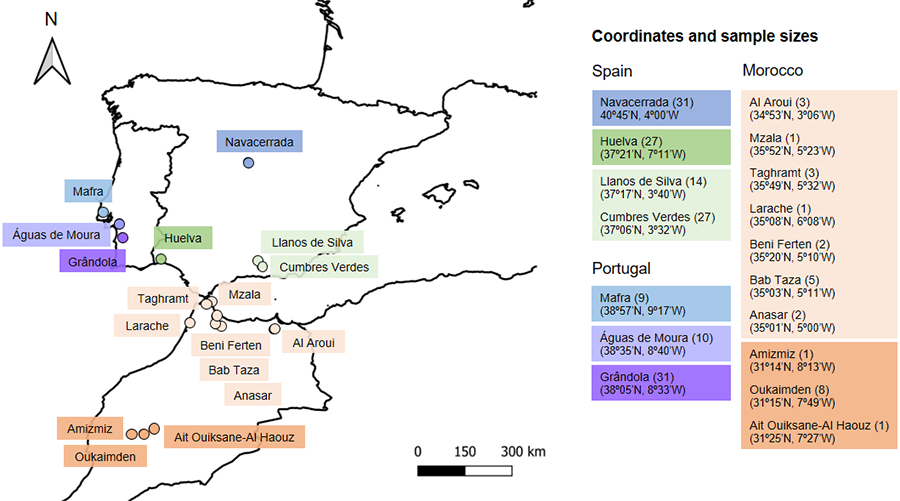
Figure 1. Map showing the geographic locations where Psammodromus algirus
lizards were sampled in Spain, Portugal and Morocco. The colours represent the
groups that were used for the statistical analyses, based on the geographic
proximity of locations and the number of captured lizards per each location. Sample
size is indicated in parentheses.
Figura 1. Mapa
mostrando las localidades geográficas donde se capturaron y muestrearon las
lagartijas Psammodromus algirus en España, Portugal y Marruecos. Los
colores representan los grupos que se usaron en los análisis estadísticos,
basándose en la proximidad geográfica de las localidades y en el número de
lagartijas capturadas en cada localidad. El tamaño de muestra
se indica entre paréntesis.
All lizards were carefully inspected for
ticks, paying special attention to the axillae, the base of the tails, skin
pockets, and the tympani, the body parts where ticks typically attach (Salvador et al. 1999; Dudek et al. 2016). Ticks were counted, removed from the lizards’ body with tweezers,
and placed in plastic tubes. Once in the laboratory, the ticks were identified
according to morphological characteristics at the stereomicroscope using
identification taxonomic keys (Pérez-Eid
2007). We measured the lizards’ snout to vent
length (SVL) with a metal ruler (to the nearest 1 mm) and weighed with a Pesola
using a polyester mesh bag or a digital scale to the nearest 0.1 g (in
Portugal, Granada and Madrid), or the nearest 0.01 g (in Huelva and Morocco).
All lizards were classified as juveniles or adults based on the presence of
nuptial colouration (absent in juveniles; Salvador et al. 1997) and SVL (cut-off
values based on the development stage of the secondary sexual characters vary
among populations and we used: 42 mm for Granada (Iberian eastern clade), 50
and 55 mm for Huelva and Portugal (Iberian western clade), and 39 mm for
Morocco; Salvador 2015). Adults were sexed according to femoral pore development, since
pores are more developed in males, especially during the breeding season (Blasco 1975; Iraeta et al. 2011). All lizards were released within a few minutes after the
manipulation, in the same place where they were captured to minimise associated
costs. We avoided recapturing the lizards by marking them either with white
paint on their back or because we collected a tail tip (see below).
Haemoglobin concentration and
leucocyte counts
We used sterile scalpel blades to collect a
tail tip of 1 cm for other purposes not reported in this study, and we used a
drop of blood from this wound to make a thin blood smear, which was air-dried
in the field. Every tail tip was then disinfected with chlorhexidine. Tail tip
removal following this procedure has no short-term negative effects on lizards
(García-Muñoz et al. 2011). In Madrid, we collected < 5 µL of peripheral blood from the
coccygeal vein using individual sterile needles (BD microlance 3, 23G, 0.6 × 25
mm) and Na-heparinized microcapillaries for haematocrit to make thin blood
smears. A second blood droplet was immediately used to quantify total
haemoglobin (Hb) concentration (g/dL) with a protocol already used in lacertids
(Megía-Palma et al. 2020b). We put the blood droplet in a disposable cuvette that was
inserted in a medical photometer (HemoCue®, Hb201+, Ängelholm, Sweden).
In the laboratory, smears were fixed in
methanol for 7 min and then stained with Giemsa diluted 1:10 in distilled water
for 45 min, except smears from Granada, Huelva, and Madrid, which were stained
with a Wright-Giemsa combination stain (RAL Diff-Quik stain set, RAL
Diagnostics and Siemens Healthineers). Smears were prepared with Eukitt®
mounting medium and were examined in a Leica DM1000LED microscope, equipped
with a camera Leica ICC50W, at 400 × magnification. We photographed 35-40
fields per blood smear and identified and counted leucocytes following Campbell and Ellis (2013). Erythrocytes were counted with the software Mizutama (Ochoa et al. 2019), which automatically counts erythrocytes according to the
identification parameters. The
relative leucocyte concentration was thus estimated as the number of leucocytes
(total number of leucocytes and leucocyte types: heterophils and lymphocytes)
per 5000 erythrocytes. We calculated the heterophil-to-lymphocyte (H/L) ratio
by dividing the number of heterophils by the number of lymphocytes. This ratio
is an indicator of physiological stress in vertebrates (Johnstone et al. 2012).
Additionally, we estimated the
relative intensity of infection by blood parasites of the suborder Adeleorina
(Apicomplexa) for each lizard host as the number of blood parasites per 5000
erythrocytes. These parasites are distinguished from others that may also be
present in the blood of western Mediterranean lacertids according to
morphological traits, and the cell membrane distortion in the infected
erythrocyte (Telford 2008; Campbell and
Ellis 2013; Megía-Palma et al. 2023). All metrics
done on the blood smears were performed by a single researcher (J.G.-B.).
Statistical analyses
We inspected for the existence of potential
outliers in every variable using Cleveland plots, and we graphically inspected
the normality and homoscedasticity of the variables (Zuur et al. 2010). One lizard from
Madrid harboured 82 ticks (CI-95% = 8.76–15.36 ticks per lizard, n = 66), a
value above the maximum reported for the population of P. algirus in
Navacerrada (range = 0–58 ticks per lizard; Carbayo et al. 2019). Unpublished data
revealed maxima of ca. 70 ticks per adult lizard in Navacerrada (Civantos,
pers. observ.). As this potential outlier seems to be in the range of this
population, we report here the analyses using the original dataset. The
analyses of intensity of tick infestation using the dataset without this lizard
are reported in the Appendix. The number of ticks was
log-transformed when it was included both as dependent and independent variable
in the models, as well as the total number of leucocytes and H/L ratio, to meet
the parametric assumptions (i.e. residual normality and homogeneity of
variances) of the subsequent linear models and linear mixed models.
We used a chi-square test to assess
variation in tick prevalence across geographic locations (eight levels: Mafra,
Águas de Moura, Grândola, Granada, Huelva, Madrid, North Morocco and South
Morocco), life stage (two levels: juveniles and adults), and sex (two levels:
male and female). Because juveniles were not sexed, we used two separate linear
models to examine variation in the intensity of tick infestation (i.e. the
number of ticks on infested hosts) with sex and life stage. The first linear
model (sex model) included the infestation intensity as the dependent variable,
and geographic location and sex as factors. The second linear model (life stage
model) included the infestation intensity as a dependent variable, and
geographic location and life stage as factors.
To analyse the relationship
between the number of leucocytes and the H/L ratio with the tick prevalence
(infested/non-infested lizards), we used the backward model selection approach
based on the Akaike Information Criterion corrected for small sample sizes
(AICc). Model selection was applied separately for the total number of
leucocytes and the H/L ratio, which were included as dependent variables in
separate models. The starting full model for these two dependent variables
included sex of the host, tick prevalence and the interaction between sex and
tick prevalence as fixed factors, SVL and number of blood parasites as
covariates, and geographic location (six levels: Mafra, Águas de Moura,
Grândola, Madrid, North Morocco and South Morocco) as a random factor, with
random intercept, hence controlling for geographic variation. Random factors
were not excluded in the backward selection process.
To analyse the relationship
between the number of leucocytes and the H/L ratio with the intensity of tick
infestation, we also used backward model selection based on AICc. In this case,
we used only tick-infested lizards and the full models for both the total
number of leucocytes and the H/L ratio included the same predictors and
interaction but included the intensity of tick infestation as predictor instead
of tick prevalence.
To examine the relationship
between haemoglobin concentration and tick infestation, we ran a linear model
using the lizard population investigated in Madrid (male-only). Total Hb
concentration was the dependent variable, and the number of ticks and SVL were
included as covariates.
We also analysed the relationship
between tick prevalence (infested/non-infested lizards) and infection
(infected/uninfected lizards) by the blood parasites, that is whether a lizard
infested by ticks was more or less likely to be infected by blood parasites,
using a Schluter’s variance ratio test (Schluter 1984).
The comparison of body size (SVL) between
life stages was checked using the t-test. The significance of models was
evaluated with an F-test using a type-III Anova, and the significance of
parameters was evaluated with a t-test. Means ± standard error (s.e.)
are presented. All analyses were performed using SPSS v 28.0 software (IBM Corp. 2021).
The data are available in the Appendix.
Results
Variation in tick infestation
A total of 66 out of 172 captured lizards
were infested by ticks (prevalence: 38.4%). The prevalence of ticks varied
significantly among geographic locations (χ27 = 110.86, P
< 0.001; Table 1). In the South of Spain (Granada and Huelva provinces), no lizards
were infested with ticks, whilst in the single site analysed in the Centre of
Spain (Madrid) 100% of the lizards were infested. Intermediate values of
prevalence were found in Portugal and Morocco (Table 1). All ticks collected in the
Iberian Peninsula (Portugal and Spain), which included 50 larvae and 79 nymphs,
were morphologically identified as I. ricinus. In the P. algirus
captured in Morocco, 11 larvae and 12 nymphs morphologically identified as I.
ricinus were detected, but also 13 ticks of the genus Hyalomma (12
larvae and 1 nymph) were found on one lizard in the North of Morocco (in
Larache), and 4 nymphs of the genus Haemaphysalis were found on two
lizards in the South of Morocco (in Oukaimden).
The prevalence of tick infestation did not
significantly differ between juveniles and adults (infested juveniles: 15 out
of 50; infested adults: 51 out of 122; χ2 = 2.09, P = 0.148).
Overall, males were more frequently infested by ticks than females (infested
males: 44 out of 92; infested females: 10 out of 44; χ2 = 7.83, P
= 0.005), but no significant differences appeared when we excluded the
male-only population of the Centre of Spain (infested males: 14 out of 62;
infested females: 10 out of 44; χ2 <
0.01, P = 0.986).
The
mean tick infestation intensity was 12.06 ± 1.65 (range = 1–82; n = 66). The
number of ticks in infested lizards varied between geographic locations, with
lizards from the Centre of Spain showing the highest intensity of infestation (sex
model: F5, 54 = 9.40, P < 0.001; life stage model: F5,
66 = 14.82, P < 0.001; Table 1). The intensity of infestation did not vary with
sex (sex model: F1, 54 = 1.04, P = 0.312), but did with life
stage (life stage model: F1, 66 = 5.02, P = 0.029). Infested
adults harboured more ticks than infested juveniles (adults: 14.80 ± 1.98,
juveniles: 2.73 ± 0.46; estimate = -0.23 ± 0.10, reference category = adults, t
= -2.24, P = 0.029), with adults being significantly larger than
juveniles (SVL adults: 71.06 ± 1.05 mm, SVL juveniles: 45.75 ± 1.72 mm; t64
= -11.77, P < 0.001).
Table 1. Prevalence of ticks (%) and intensity of tick infestation in Psammodromus
algirus lizards in the Iberian Peninsula and Morocco. The number of lizards
captured is shown in parentheses. Means and standard errors are shown.
Tabla 1. Prevalencia
(%) e intensidad de infestación por garrapatas en la lagartija Psammodromus
algirus en la Península Ibérica y Marruecos. El número de lagartijas
capturadas se muestra entre paréntesis. Las medias se muestran con su error
estándar.
Tick infestation and host
leucocyte counts
The selected model analysing the
relationship between tick prevalence and the total number of leucocytes
retained the prevalence of ticks and SVL as predictors, but only the SVL
significantly covaried with the number of leucocytes. The larger the lizard,
the lower the number of leucocytes (Table 2). The selected model
analysing the relationship between tick infestation intensity and the total
number of leucocytes retained solely the tick intensity as a covariate, with
infested lizards with more ticks having fewer leucocytes (Table 2).
However, this significant association disappeared when we excluded from the
dataset the lizard specimen harbouring 82 ticks (see Appendix).
The selected model analysing the
association between tick prevalence and the H/L ratio retained host sex, tick
prevalence, and the interaction between sex and tick prevalence as predictors,
all significantly associated with the H/L ratio. Non infested males showed a
significantly higher H/L ratio than non-infested females, however infested
males had a significantly reduced H/L ratio compared to non-infested males,
while infested females displayed a similar H/L ratio to non-infested females (Fig. 2; Table 2).
The selected model analysing the association between tick infestation intensity
and the H/L ratio retained solely sex of the host as predictor. As revealed
from the aforementioned model, infested females showed a higher H/L ratio than
males (Table 2).
Tick infestation intensity and
haemoglobin concentration
The mean Hb concentration in tick-infested
male lizards was 11.06 ± 0.35 g/dL (range = 7.30–15.40; n = 30). Total Hb
concentration did not significantly vary with tick intensity (F1, 30
= 0.36, P = 0.556) or SVL (F1, 30 = 2.27, P = 0.143).
Association between tick
infestation and blood parasites
18.6% (29/156) of the lizards were infected
with blood parasites. Mean intensity of these blood parasites was 11.55 ± 2.11
(range = 1–44; n = 29). We found no association between tick infestation status
and infection by blood parasites (Schluter test: variance ratio = 0.58, W =
88.38, d.f. = 151, Wc = 181.77).
Table 2. Results of the selected linear mixed-effects models for the
variation in the total number of leucocytes and the H/L ratio in Psammodromus
algirus lizards. Prevalence models were run considering all lizards, and
intensity models were run considering tick-infested lizards. The dependent
variables were log-transformed. The significant parameters (P < 0.05)
are marked in bold.
Tabla 2.
Resultados de los modelos lineales de efectos mixtos seleccionados para la
variación en el número total de leucocitos y ratio H/L en la lagartija Psammodromus
algirus. Los modelos de prevalencia se llevaron a cabo usando todas las
lagartijas, mientras que en los modelos de intensidad se usaron solo las
lagartijas infestadas por garrapatas. Las variables dependientes se
transformaron logarítmicamente. Los parámetros significativos (P <
0.05) se marcan en negrita.
Note: in all models, the reference categories for the
sex and prevalence are females and 1 (infested).
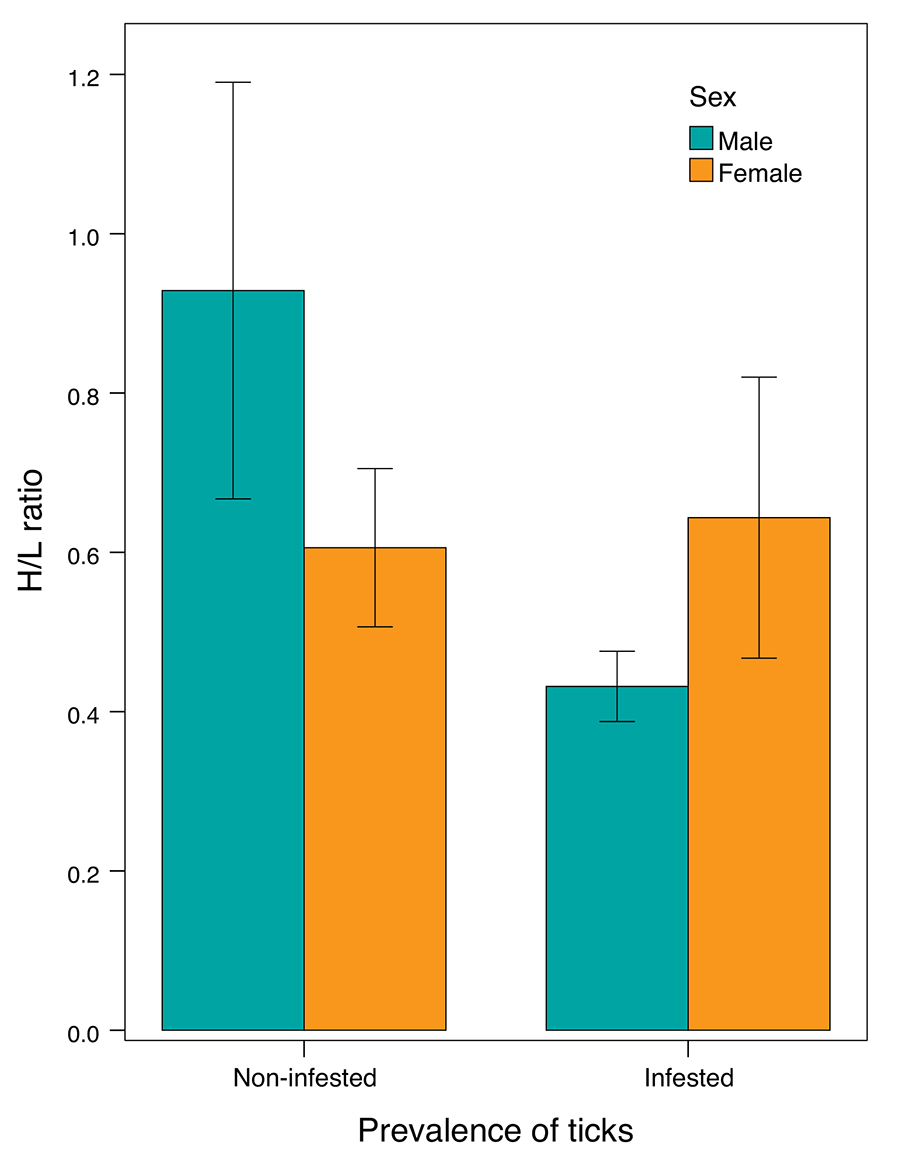
Figure 2. Variation in the heterophil-to-lymphocyte ratio (H/L ratio) between
sexes and tick infestation status in P. algirus hosts. The bars show the
± standard error of the mean. Plot show the raw data, but the analyses were
carried out with the dependent variable log-transformed.
Figura 2. Variación en la ratio
heterófilo-linfocito (ratio H/L) entre sexos y estado de infestación por
garrapatas en la lagartija Psammodromus algirus. Las barras muestran el
error estándar de la media. La figura muestra los datos sin transformar, pero
los análisis se realizaron con la variable dependiente transformada mediante
logaritmo (log10).
Discussion
We examined the geographic variation of
tick infestation in the lizard P. algirus along most of its geographic
distribution, the Iberian Peninsula and North Africa. The mean prevalence of
ticks in P. algirus was 38%, although we found a high variation among
locations. Environmental conditions for ticks and lizard hosts vary
geographically and may affect the intensity of ectoparasite infestation. In the
current study, no lizard was infested with ticks in the South of Spain (but see
Hernández-Rojas et al.
2025). Although ticks are rarely found in
populations of P. algirus in central Spain, all the lizards from the
population here investigated in Madrid were infested. Intermediate prevalence
scores were found in Morocco (30%) and Portugal (north of Tagus river: 90%,
south of Tagus river: 10-60%). These findings reveal that tick prevalence
widely varies among populations of P. algirus, probably linked to
differences in exposure due to environmental conditions and densities of
reproduction and alternative hosts (Álvarez-Ruiz et al. 2018; Carbayo et al. 2019; Peralbo-Moreno
et al. 2022; Hernández-Rojas et al. 2025).
The life cycle of I. ricinus depends
on a broad range of hosts on which immature stages and males feed. But
reproduction and population maintenance require a narrower group of hosts
(large vertebrates, mainly ungulates) on which females feed (Gray et al. 2009).
The adult stage of the other tick genera (Hyalomma and Haemaphysalis)
found in this study also depends on large vertebrates (Hoogstraal 1955; 1971; Apanaskevich
et al. 2007; Sajid et al. 2018). Hence, the observed
geographical variation in tick infestation in P. algirus may likely be
due to differences in the abundance of appropriate hosts for these ticks (large
carnivores and ungulates), since lizards act only as intermediate hosts in
which adult ticks are not found. Indeed, in the north of the Tagus River in
Portugal, we recorded high infestation loads in lizards, which is likely linked
to the high densities of fallow
deer that occur in the National Hunting Ground of Tapada de Mafra. Similarly,
in the Centre of Spain, lizards harboured the highest tick load compared to
other examined locations, and a previous study conducted in the same area
(Navacerrada) found a high tick prevalence (92%) and tick infestation intensity
(12 ticks per individual) on P. algirus (Carbayo et al. 2019). This site is
composed of a deciduous forest harbouring significant numbers of ungulates,
such as roe deer, wild boars, and cattle (Carbayo et al. 2019). Thus, differences
among populations could be explained by variations in the occurrence and
abundance of large vertebrates, as these endotherms are selected by the adult
stage of Ixodes (Tack et al.
2012; Wu
et al. 2019).
Although no tick was found infesting
lizards from the South of Spain, ticks were previously found in P. algirus
from Sierra Nevada (Granada), in the southeast of Spain, but with a low
prevalence (Álvarez-Ruiz et
al. 2018; Hernández-Rojas et al. 2025). Therefore,
it is unlikely that climatic conditions in Sierra Nevada are extremely severe
for this ectoparasite, given the environmental requirements of Ixodes ticks
in Spain (Megía-Palma et al.
2024a; Hernández-Rojas et al. 2025). Overall,
these results support that P. algirus is a competent host for immature
stages of I. ricinus in Europe and North Africa (Dsouli et al. 2006; Soualah-Alila
et al. 2015). These results are consistent with
previous studies (Václav et al.
2007; Dudek
et al. 2016; Tommassone et al. 2017; Megía-Palma et al. 2018; Wu et al. 2019). Although only in three of the lizards, we also found larvae and
nymph tick stages of the hard tick Hyalomma and Haemaphysalis
infesting P. algirus in Morocco.
We explored the relationships
between the cellular components of the immune system, blood physiology, and
tick intensity. Based on previous studies on lizards and other vertebrate
models, we expected that tick-infested P. algirus would have shown low
levels of haemoglobin and altered leucocyte counts due to either (a) a
decreased immunocompetence in infested lizards (Veiga et al. 1998), or (b) direct tick
effects on immune cellular components. For example, in blackbirds (Turdus
merula), tick-infested individuals showed a higher H/L ratio than
non-infested ones, especially in cases of high infestation (Heylen and Matthysen 2008; Norte et al. 2013). However, in great tits (Parus major) experimentally
infested with ticks, no effects on leucocyte count were observed, although the
sedimentation rate increased (Heylen and Matthysen 2008). Here, we
found no significant differences in leucocyte counts between tick-infested and
non-infested lizards, whereas sexual differences in H/L ratios were
significant. Interestingly, infested females had a higher H/L ratio than
infested males. However, the H/L ratios of infested and non-infested females
were similarly high (Fig. 2), this suggested a lower immune responsiveness of males to tick
infestation because the H/L ratio in males was generally lower. This pattern
could be explained by a higher susceptibility to stress of females or because
females may invest more resources in parasite defence (Dupoué et al. 2020; Megía-Palma et
al. 2024a).
Regarding intensity of tick infestation, we
found that lizards with higher tick loads had fewer leucocytes in peripheral
blood, but this relationship only existed because of a single individual with
82 ticks Appendix; analyses without this lizard: P =
0.20). This suggests that tick load does not cause severe alterations in the
lizards’ immune physiology or that lizards were able to compensate the
infestation, at least in infestations with low to medium number of ticks. This
finding complies with studies reporting pathogenic effects of ticks beyond
certain high infestation threshold (Norte et al. 2013; but see Main and Bull 2000). Uller and
Olsson (2003) also did not detect effects of tick
infestation in blood metrics (leucocyte and erythrocyte counts) in the common
lizard (Zootaca vivipara; fam. Lacertidae). In their experimental study,
only growth rates were negatively affected by tick infestation, and that was
only in juveniles that had been exposed to testosterone in-ovo.
The effects of tick infestation may differ
among studies due to different environmental conditions that may favour or
limit the capacity of the host to compensate for resources consumed by the
ectoparasite (Megía-Palma et
al. 2024b). Main and Bull (2000) suggested that a
trade-off may exist between the fitness advantages of
occupying high-quality microhabitats and the costs of
tick parasitism occurring in these habitats. In other
studies, age has been suggested as mediator of the magnitude of the
pathogenicity associated with the ticks: wild
tick-infested blackbirds showed increased globulin concentrations, but only
those that were older than one year (Norte et al. 2013), suggesting an
acquired immune response (Jones et
al. 2015; but see Heylen et al. 2010), although
differential mortality could not be excluded as a potential explanation.
However, the number of ticks infesting male lizards did not alter their
haemoglobin concentration. In the same way, no alterations in aerobic capacity
or locomotion caused by tick infestation have been detected in other lizard
species (Ekner-Grzyb et al.
2013; Albuquerque et al. 2023; Wild and Gienger 2024). In general, it seems that ticks do not represent a strong
pressure for this lizard host, although P. algirus males reduced their
H/L ratio when infested by ticks (Fig. 2).
We expected that male lizards were more
frequently infested by ticks than females, a general pattern previously found
in several lizard species (Václav
et al. 2007; Dudek et al. 2016; but see Wu et al. 2019),
including P. algirus (Carbayo
et al. 2019). When
analysing the data from all populations, we found that males were more
frequently infested by ticks than females, in line with recent research in
several lacertid lizards (Hernández-Rojas et al. 2025). This could
be related to marked sexual dimorphism in response to infections, influenced by
the higher levels of immunosuppressive testosterone in males (Salvador et al. 1996; Mondal and Rai
1999; Belliure et al. 2004; Pollock et al. 2012). Alternatively, male lizards could invest more time foraging in,
or defending, wider home ranges than females during the breeding season (Perry and Garland 2002), which in turn increases the probability of infestation because of
the greater mobility by males (Olsson
et al. 2000). However, when we excluded the data of
the population from central Spain, where only males were analysed and all of
them were infested with I. ricinus, we did not observe significant
sexual differences in tick infestation in the restricted sample. This precludes
us from separating the effect of host sex from that of the geographic locality
on the between-sex differences in tick prevalence. Therefore, our evidence does
not support the hypothesis that males of P. algirus are more susceptible
to infestation than females of this species. We found that the prevalence of
ticks in adult lizards was not higher than in juveniles. However, adult lizards
carried more ticks on average than juveniles. This result could indicate that
larger hosts can harbour more ticks (Watkins and Blouin-Demers 2019; Ferreira et al. 2023). Since body size, rather than age, is likely the main factor
explaining this pattern, we also analysed tick intensity in relation to lizard
body size. In fact, we found a positive relationship between SVL and tick
intensity (linear model; estimate = 0.48 ± 0.11, t = 4.22, P <
0.001), supporting the idea that larger individuals sustain higher parasite
loads.
Lastly, Schluter’s variance ratio test
revealed no significant association between tick infestation and susceptibility
to infections by blood parasites. We expected that tick infestation would
facilitate blood parasite infections by increasing the susceptibility to
infestation by other arthropod vectors as a cost associated with a putative
immunosuppression effect of tick infestation (Veiga et al. 1998). In the case of P.
algirus, tick infestation could facilitate the infestation by
haematophagous mites, some of them described as vectors of blood parasites (Telford 2008; Haklová-Kočíková et al. 2014; Megía-Palma et
al. 2023). In fact, ticks and mites can co-infest
the same host with loads that can negatively correlate to each other,
suggesting they may compete (Wu et
al. 2019). The genus mite Ophionyssus (fam.
Macronyssidae) is a common ectoparasite of P. algirus across its
geographic distribution, including some of the sites examined here (Soualah-Alila et al. 2009; Álvarez-Ruiz et
al. 2018; 2021; Carbayo et al. 2019; Megía-Palma et al. 2022). Several alternative hypotheses can explain the absence of a
significant association between tick infestation and blood parasite infection:
(i) mites and other vectors of adeleorine blood parasites were not attached to
the body of tick-infested lizards at the time of capture; (ii) ticks other than
those detected may have transmitted blood parasites to lizards but detached
from their body before we captured lizards; (iii) the detected adeleorine blood
parasites may have been transmitted by other arthropod vectors not examined
here; or (iv) adeleorine blood parasites may not have reached high numbers in
the bloodstream because of a decreased merogony. These hypothetical cases
probably explain the absence of significant correlation between tick load and
blood parasite infection in lacertid lizards (Álvarez-Ruiz et al. 2018; Megía-Palma et al. 2020a). Moreover, blood parasites may be tolerated by lacertids even when
they are at low levels of body condition (Faria et al. 2024; Megía-Palma et al. 2024a).
In conclusion, our study reveals drastic
geographic variation in the prevalence and intensity of ticks infesting P.
algirus across its range. According to previous studies, extrinsic factors
not investigated here, such as the local abundance of large mammals, and
microclimatic conditions might contribute to explain the observed geographic
variation. Our findings also show no correlations between I. ricinus
tick parasitism, P. algirus haematic physiology (although males infested
with ticks had lower H/L ratio than non-infested males) and blood parasites.
The subjacent causes of the absence of the expected correlations among the
measured variables deserve further experimental investigation and/or
longitudinal analyses.
Author contributions
Jorge Garrido-Bautista: Conceptualization, Data curation, Formal analysis, Investigation,
Methodology, Visualization, Writing – Original draft. Gregorio Moreno-Rueda:
Conceptualization, Funding acquisition, Investigation, Methodology, Project
administration, Supervision, Writing – Original draft. Francisco J.
Zamora-Camacho: Data curation, Investigation, Writing – Review and editing.
Mar Comas: Conceptualization, Funding acquisition, Investigation,
Methodology, Project administration, Writing – Original draft. El-Mustapha
Laghzaoui: Investigation. Miguel A. Carretero: Investigation,
Writing – Review and editing. Afonso D. Rocha: Investigation, Writing –
Review and editing. Sofía I. Arce: Investigation, Writing – Review and
editing. Emilio Civantos: Investigation, Writing – Review and editing. Rodrigo
Megía-Palma: Investigation, Methodology, Writing – Review and editing. Luis
P. da Silva: Investigation, Writing – Review and editing. Ana Cláudia
Norte: Conceptualization, Data curation, Formal analysis, Funding acquisition,
Investigation, Methodology, Project administration, Supervision, Writing –
Original draft.
Data availability
Data are available in the supplementary
material of this article (https://doi.org/10.7818/ECOS.2931SM).
Financing, required permits, potential
conflicts of interest and acknowledgments
This study had the support of Portuguese
national funds through Fundação para a Ciência e a Tecnologia, I. P (FCT),
under the projects UIDB/04292/2020 (https://doi.org/10.54499/UIDB/04292/2020) and UIDP/04292/2020 (https://doi.org/10.54499/UIDP/04292/2020) granted to MARE, LA/P/0069/2020 (https://doi.org/10.54499/LA/P/0069/2020) granted to the Associate Laboratory ARNET, 2022.03391.PTDC (https://doi.org/10.54499/2022.03391.PTDC) granted to CIBIO, and UID Centro de Estudos do Ambiente e Mar
(CESAM) + LA/P/0094/2020 granted to CESAM. The study also had the support of
the research contract (CEECIND/02064/2017: https://doi.org/10.54499/CEECIND/02064/2017/CP1423/CP1645/CT0009) to L.P.S., and transitory norm contracts (DL57/2016/CP1370/CT89)
to A.C.N. The sampling in southern Spain was economically supported by a grant
from the Spanish Society for Ethology and Evolutionary Ecology, granted to M.C.
F.J.Z.-C. was partly supported by a Juan de la Cierva-Incorporación
postdoctoral fellowship by the Spanish Ministry of Economy, Industry and
Competitiveness.
All lizards were captured and sampled
according to each national legislation and with the corresponding permits.
Sampling in Portugal was carried out with the permission issued by Instituto da
Conservação da Natureza e das Florestas (reference: 111/2022/CAPT). Sampling in
Morocco was carried out under the permission from L’Agence Nationale des Eaux
et Forêts (ANEF) (reference: N#372/2020). Sampling in Madrid was carried out
under the licence granted by the Dirección General de Biodiversidad y Recursos
Naturales, Comunidad Autónoma de Madrid, Spain (reference: 10/439587.9/23).
Sampling in south Spain was carried out with the permission for animal
experimentation issued by the Andalusian government to G.M.R. (reference:
04/02/2019/012).
The authors declare that they have no
conflict of interest.
We thank the COST Action CA21170
“Prevention, anticipation and mitigation of tick-borne diseases risk applying
the DAMA protocol – PRAGMATICK” for promoting networking among researchers on
ticks and tick-borne diseases that contributed to share ideas on the topic of
this manuscript. Pablo Melero-Romero and Jesús Arca helped us capturing
lizards in Granada.
References
Albuquerque, R.L., Zani, P.A.,
Garland, T. 2023. Lower-level predictors and behavioral
correlates of maximal aerobic capacity and sprint speed among individual
lizards. Journal of Experimental Biology 226(5), jeb244676. https://doi.org/10.1242/jeb.244676
Álvarez-Ruiz, L., Megía-Palma,
R., Reguera, S., Ruiz, S., Zamora-Camacho, F.J., Figuerola, J., Moreno-Rueda,
G. 2018. Opposed elevational variation in prevalence and
intensity of endoparasites and their vectors in a lizard. Current Zoology
64(2), 197-204. https://doi.org/10.1093/cz/zoy002
Álvarez-Ruiz, L., Belliure, J., Santos, X., Pausas, J.G. 2021. Fire reduces
parasite load in a Mediterranean lizard. Proceedings of the Royal Society B:
Biological Sciences 288, 20211230. https://doi.org/10.1098/rspb.2021.1230
Amo, L., López, P., Martín, J. 2007. Habitat deterioration affects antipredatory behavior, body
condition, and parasite load of female Psammodromus algirus lizards. Canadian
Journal of Zoology 85(6), 743-751. https://doi.org/10.1139/Z07-052
Apanaskevich, D.A., Horak, I.G., Camicas, J.L. 2007. Redescription of Haemaphysalis
(Rhipistoma) elliptica (Koch, 1844), an old taxon of the Haemaphysalis (Rhipistoma)
leachi group from East and southern Africa, and of Haemaphysalis
(Rhipistoma) leachi (Audouin, 1826) (Ixodida, Ixodidae). Journal
of Veterinary Research 74(3), 181-208. https://doi.org/10.4102/ojvr.v74i3.122
Arakelyan, M., Harutyunyan, T., Aghayan, S.A., Carretero, M.A. 2019.
Infection of parthenogenetic lizards by blood parasites does not support the
“Red Queen hypothesis” but reveals the costs of sex. Zoology 136,
125709. https://doi.org/10.1016/j.zool.2019.125709
Belliure, J., Smith, L., Sorci, G. 2004. Effect of testosterone on T
cell-mediated immunity in two species of Mediterranean lacertid lizards. Journal
of Experimental Zoology A: Comparative Experimental Biology 301A(5),
411-418. https://doi.org/10.1002/jez.a.20068
Blasco, M. 1975. El dimorfismo
sexual en cinco especies de la familia Lacertidae (Reptilia). Boletín de la
Real Sociedad Española de Historia Natural 73, 237-242.
Bull, C.M., Burzacott, D.
1993. The impact of tick load on the fitness of their lizard
hosts. Oecologia 96(3), 415-419. https://doi.org/10.1007/BF00317513
Campbell,
T.W., Ellis, C.K. (eds.) 2013. Avian and exotic
animal hematology and cytology. Wiley, London, United Kingdom.
Carbayo, J., Martín, J., Civantos, E. 2019. Habitat type influences
parasite load in Algerian Psammodromus (Psammodromus algirus) lizards. Canadian
Journal of Zoology 97(2), 172-180. https://doi.org/10.1139/cjz-2018-0145
Comas, M., Zamora-Camacho, F.J.,
Garrido-Bautista, J., Moreno-Rueda, G., Martín, J., López, P. 2025. Mounting an immune response reduces male attractiveness in a lizard.
Integrative Zoology 20(4), 728-739. https://doi.org/10.1111/1749-4877.12889
Drechsler, R.M., Belliure, J., Megía-Palma, R. 2021. Phenological and
intrinsic predictors of mite and haemacoccidian infection dynamics in a
Mediterranean community of lizards. Parasitology 148(11), 1328-1338. https://doi.org/10.1017/S0031182021000858
Dsouli, N., Younsi-Kabachii, H., Postic, D., Nouira S., Gern, L.,
Bouattour, A. 2006. Reservoir role of lizard Psammodromus algirus in
transmission cycle of Borrelia burgdorferi sensu lato (Spirochaetaceae)
in Tunisia. Journal of Medical Entomology 43(4), 737-742. https://doi.org/10.1093/jmedent/43.4.737
Dudek, K., Skórka, P., Sajkowska, Z.A., Ekner-Grzyb, A., Dudek, M.,
Tryjanowski, P. 2016. Distribution pattern and number of ticks on lizards. Ticks
and Tick-borne Diseases 7(1), 172-179. https://doi.org/10.1016/j.ttbdis.2015.10.014
Dupoué A., Blaimont P., Rozen-Rechels D., Richard M., Meylan S., Clobert
J., Miles D.B., et al. 2020. Water availability and temperature induce changes
in oxidative status during pregnancy in a viviparous lizard. Functional
Ecology 34(2), 475-485. https://doi.org/10.1111/1365-2435.13481
Ekner-Grzyb, A., Sajkowska, Z., Dudek, K., Gawałek, M., Skórka, P.,
Tryjanowski, P. 2013. Locomotor performance of sand lizards (Lacerta agilis):
effects of predatory pressure and parasite load. Acta Ethologica
16, 173-179. https://doi.org/10.1007/s10211-013-0148-2
Faria, J.F., Megía-Palma, R.,
Harris, D.J. 2024. Impact of blood parasites on the behaviour
of two congeneric wall lizards (genus Podarcis). Behavioral Ecology
and Sociobiology 78, 105. https://doi.org/10.1007/s00265-024-03518-8
Ferreira, A.I., Damas-Moreira, I., Marshall, K.L.A., Perera, A., Harris, J.
2023. What influences the prevalence and intensity of haemoparasites and
ectoparasites in an insular lizard? Animals 13(4), 723. https://doi.org/10.3390/ani13040723
García-Muñoz,
E., Sillero, N. 2010. Two
new types of noose for capturing herps. Acta Herpetologica 5(2),
259-264.
García-Muñoz, E., Ceacero, F.,
Pedrajas, L., Kaliontzopoulou, A., Carretero, M.A. 2011. Tail
tip removal for tissue sampling has no short-term effects on microhabitat
selection by Podarcis bocagei, but induced autotomy does. Acta
Herpetologica 6(2), 223-227.
Gray, J.S., Dautel, H., Estrada-Peña,
A., Kahl, O., Lindgren, E. 2009. Effects of climate change on
ticks and tick-borne diseases in Europe. Interdisciplinary Perspectives on
Infectious Diseases 2009(1), 593232. https://doi.org/10.1155/2009/593232
Haklová-Kočíková, B., Hižňanová, A., Majláth, I., Račka, K., Harris, D.J., Földvári,
G., Tryjanowski, P., et al. 2014. Morphological and molecular characterization
of Karyolysus – a neglected but common parasite infecting some European
lizards. Parasites & Vectors 7, 555. https://doi.org/10.1186/PREACCEPT-6079415861356224
Hernández-Rojas, C., Olmeda, A.S., Valcárcel, F., Sánchez, M., Fitze, P.S., Reguera,
S., Moreno-Rueda, G., et al. 2025. Contribución de los lacértidos a la
distribución de las garrapatas en la península ibérica. Ecosistemas
34(2), 2922. https://doi.org/10.7818/ECOS.2922
Heylen, D.J.A., Matthysen,
E. 2008. Effect of tick parasitism on the health status of a
passerine bird. Functional Ecology 22(6), 1099-1107. https://doi.org/10.1111/j.1365-2435.2008.01463.x
Heylen, D.J.A., Madder, M., Matthysen, E. 2010. Lack of resistance against
the tick Ixodes ricinus in two related passerine bird species.
International Journal for Parasitology 40(2), 183-191. https://doi.org/10.1016/j.ijpara.2009.07.011
Hoogstraal, H. 1955. Notes on African Haemaphysalis ticks. I. The
Mediterranean-littoral hedgehog parasite H. erinacei Pavesi, 1884
(Ixodoidea, Ixodidae). Journal of Parasitology 41(3), 221-233. https://doi.org/10.2307/3274195
Hoogstraal, H. 1971. Identity, hosts, and distribution of Haemaphysalis
(Rhipistoma) canestrinii (Supino) (Resurrected), the postulated
Asian progenitor of the African Leachi complex (Ixodoidea, Ixodidae). Journal
of Parasitology 57(1), 161-172. https://doi.org/10.2307/3277774
IBM Corp 2021. IBM SPSS Statistics for Windows. Version 28.0. Released 2021.
Armonk, New York, USA. https://www.ibm.com/es-es/products/spss
Iraeta, P., Monasterio, C.,
Salvador, A., Díaz, J.A. 2011. Sexual dimorphism and
interpopulation differences in lizard hind limb length: locomotor performance
or chemical signalling? Biological Journal of the Linnean Society
104(2), 318-329. https://doi.org/10.1111/j.1095-8312.2011.01739.x
Johnstone, C.P., Reina, R.D., Lill, A. 2012. Interpreting indices of
physiological stress in free-living vertebrates. Journal of Comparative
Physiology B: Biochemical, Systemic, and Environmental Physiology 182,
861-879. https://doi.org/10.1007/s00360-012-0656-9
Jones, C.R., Brunner, J.L., Scoles, G.A., Owen, J.P. 2015. Factors
affecting larval tick feeding success: host, density and time. Parasites
& Vectors 8, 340-340. https://doi.org/10.1186/s13071-015-0955-6
Kahl, O., Gray, J.S. 2023. The biology of Ixodes ricinus with emphasis on
its ecology. Ticks and Tick-borne Diseases 14(2), 102114. https://doi.org/10.1016/j.ttbdis.2022.102114
Kar, S., Rodríguez, S.E., Akyildiz, G., Cajimat, M.N.B., Bircan, R.,
Mears, M.C., Bente, D.A., et al. 2020. Crimean-Congo hemorrhagic fever virus in
tortoises and Hyalomma aegyptium ticks in east Thrace, Turkey: potential
of a cryptic transmission cycle. Parasites & Vectors 13, 201. https://doi.org/10.1186/s13071-020-04074-6
Knapp, C.R., Pérez-Heydrich, C., Zachariah, T.T., Jollay, J., Schnelle,
A.N., Buckner, S.D., Lattin, C.R., et al. 2019. Host sex, size, and
hemoparasite infection influence the effects of ectoparasitic burdens on
free-ranging iguanas. Ecology & Evolution 9(4), 1946-1956. https://doi.org/10.1002/ece3.4887
Lanser, D.M., Vredevoe, L.K., Kolluru, G.R. 2021. Tick parasitism impairs
contest behavior in the western fence lizard (Sceloporus occidentalis). Behavioral
Ecology and Sociobiology 75(2), 40. https://doi.org/10.1007/s00265-021-02980-y
Lopes de
Carvalho, I., Fonseca, J.E., Marques, J.G.,
Ullmann, A., Hojgaard, A., Zeidner, N., Núncio, M.S. 2008. Vasculitis-like
syndrome associated with Borrelia lusitaniae infection. Clinical
Rheumatology 27, 1587-1591. https://doi.org/10.1007/s10067-008-1012-z
Main, A.R., Bull, C.M. 2000. The impact of tick parasites on the behaviour of the
lizard Tiliqua rugosa. Oecologia 122(4), 574-581. https://doi.org/10.1007/s004420050981
Martín, J., Civantos, E., Amo, L.,
López, P. 2007. Chemical ornaments of male lizards Psammodromus
algirus may reveal their parasite load and health state to females. Behavioral Ecology and Sociobiology 62,
173-179. https://doi.org/10.1007/s00265-007-0451-x
Megía-Palma, R., Martínez, J., Merino, S. 2018. Manipulation
of parasite load induces significant changes in the structural-based throat
color of male Iberian green lizards. Current Zoology 64(3),
293-302. https://doi.org/10.1093/cz/zox036
Megía-Palma, R., Arregui, L.,
Pozo, I., Žagar, A., Serén, N., Carretero, M.A., Merino, S. 2020a. Geographic patterns of stress in insular lizards reveal
anthropogenic and climatic signatures. Science
of the Total Environment 749, 141655. https://doi.org/10.1016/j.scitotenv.2020.141655
Megía-Palma, R.,
Jiménez-Robles, O., Hernández-Agüero, J.A., De la Riva, I. 2020b. Plasticity of haemoglobin concentration and thermoregulation in a
mountain lizard. Journal of Thermal Biology 92, 102656. https://doi.org/10.1016/j.jtherbio.2020.102656
Megía-Palma, R., Paranjpe, D., Blaimont, P., Cooper, R., Sinervo, B. 2020c. To cool or not to cool? Intestinal coccidians disrupt the
behavioural hypothermia of lizards in response to tick infestation. Ticks
and Tick-borne Diseases 11(1), 101275. https://doi.org/10.1016/j.ttbdis.2019.101275
Megía-Palma, R., Merino, S.,
Barrientos, R. 2022. Longitudinal effects of habitat quality,
body condition, and parasites on colour patches of a multiornamented lizard. Behavioral
Ecology and Sociobiology 76, 73. https://doi.org/10.1007/s00265-022-03182-w
Megía-Palma, R., Martínez, J.,
Fitze, P.S., Cuervo, J.J., Belliure, J., Jiménez-Robles, O., Cabido, C., et al.
2023. Genetic diversity, phylogenetic position, and
co-phylogenetic relationships of Karyolysus, a common blood parasite of
lizards in the western Mediterranean. International Journal for
Parasitology 53, 185-196. https://doi.org/10.1016/j.ijpara.2022.12.006
Megía-Palma, R., Cuervo, J.J.,
Fitze, P.S., Martínez, J., Jiménez-Robles, O., De la Riva, I., Reguera, S., et
al. 2024a. Do sexual differences in life strategies make male
lizards more susceptible to parasite infection? Journal of Animal Ecology 93(9),
1338-1350. https://doi.org/10.1111/1365-2656.14154
Megía-Palma, R., Paranjpe, D., Cooper, R.D., Blaimont, P., Sinervo, B. 2024b.
Natural parasites in conjunction with behavioral and color traits explain male
agonistic behaviors in a lizard. Current Zoology 70(1), 59-69. https://doi.org/10.1093/cz/zoac095
Mendoza-Roldan, J.A.,
Mendoza-Roldan, M.A., Otranto, D. 2021. Reptile vector-borne
diseases of zoonotic concern. International Journal for Parasitology:
Parasites and Wildlife 15, 132-142. https://doi.org/10.1016/j.ijppaw.2021.04.007
Mondal, S., Rai, U. 1999. Sexual dimorphism in phagocytic activity of wall lizard’s
splenic macrophages and its control by sex steroids. General and Comparative
Endocrinology 116(2), 291-298. https://doi.org/10.1006/gcen.1999.7370
Norte, A.C., Lopes de Carvalho, I., Ramos, J.A., Gonçalves, M., Gern, L.,
Núncio, M.S. 2012. Diversity and seasonal patterns of ticks parasitizing wild
birds in western Portugal. Experimental and Applied Acarology 58,
327-339. https://doi.org/10.1007/s10493-012-9583-4
Norte, A.C., Lobato, D.N.C., Braga, E.M., Antonini, Y., Lacorte, G.,
Gonçalves, M., Lopes de Carvalho, I., et al. 2013. Do ticks and Borrelia
burgdorferi s.l. constitute a burden to birds? Parasitology
Research 112, 1903-1912. https://doi.org/10.1007/s00436-013-3343-1
Norte, A.C., Alves da Silva, A.,
Alves, J., Pascoal da Silva, L., Núncio, M.S., Escudero, R., Anda, P., et al.
2015. The importance of lizards and small mammals as
reservoirs for Borrelia lusitaniae in Portugal. Environmental
Microbiology 7(2), 188-193. https://doi.org/10.1111/1758-2229.12218
Ochoa, D., Redondo, T.,
Moreno-Rueda, G. 2019. Mizutama: a quick, easy, and accurate
method for counting erythrocytes. Physiological and Biochemical Zoology
92(2), 206-210. https://doi.org/10.1086/702666
Olsson, M., Wapstra, E., Madsen, T., Silverin, B. 2000. Testosterone,
ticks and travels: a test of the immunocompetence-handicap hypothesis in
free-ranging male sand lizards. Proceedings of the
Royal Society B: Biological Sciences 267,
2339-2343. https://doi.org/10.1098/rspb.2000.1289
Peralbo-Moreno, A.,
Baz-Flores, S., Cuadrado-Matías, R., Barroso, P., Triguero-Ocaña, R.,
Jiménez-Ruiz, S., Herraiz, C., et al. 2022. Environmental
factors driving fine-scale ixodid tick abundance patterns. Science of the
Total Environment 853, 158633. https://doi.org/10.1016/j.scitotenv.2022.158633
Pérez-Eid, C. 2007. Les tiques: identification, biologie, importance
médicale et veterinaire. Monographies de microbiologie. Éd. Tec & Doc /
Lavoisier, Paris, France.
Perry, G.,
Garland, Jr., T. 2002. Lizard home ranges
revisited: effects of sex, body size, diet, habitat, and phylogeny. Ecology 83(7),
1870-1885. https://doi.org/10.1890/0012-9658(2002)083[1870:LHRREO]2.0.CO;2
Pfaffl, M., Petney, T., Elgas, M., Skuballa, J., Taraschewski, H. 2009.
Tick-induced blood loss leads to regenerative anaemia in the European hedgehog
(Erinaceus europaeus). Parasitology 136(4), 443-452. https://doi.org/10.1017/S0031182009005514
Pollock, N.B., Vredevoe, L.K.,
Taylor, E.N. 2012. The effect of exogenous testosterone on
ectoparasite loads in free-ranging western fence lizards. Journal of
Experimental Zoology A: Ecological and Integrative Physiology 317(7),
447-454. https://doi.org/10.1002/jez.1737
Rodgers,
M.L., Bolnick, D.I. 2024. Opening a can of worms: a
test of the co-infection facilitation hypothesis. Oecologia 204,
317-325. https://doi.org/10.1007/s00442-023-05409-7
Sajid, M.S., Kausar, A., Iqbal, A.,
Abbas, H., Iqbal, Z., Jones, M.K. 2018. An insight into the
ecobiology, vector significance and control of Hyalomma ticks (Acari:
Ixodidae): a review. Acta Tropica 187, 229-239. https://doi.org/10.1016/j.actatropica.2018.08.016
Salvador, A. 2015. Lagartija
colilarga – Psammodromus algirus. En: Enciclopedia Virtual de los
Vertebrados Españoles. Salvador, A., Marco, A. (Eds.). Museo Nacional de
Ciencias Naturales (MNCN-CSIC), Madrid. http://www.vertebradosibericos.org/
[Accessed on June 2025].
Salvador, A., Veiga, J.P.,
Martín, J., López, P., Abelenda, M., Puerta, M. 1996. The cost
of producing a sexual signal: testosterone increases the susceptibility of male
lizards to ectoparasitic infestation. Behavioral Ecology 7(2), 145-150. https://doi.org/10.1093/beheco/7.2.145
Salvador, A., Veiga, J.P., Martín, J., López, P. 1997. Testosterone supplementation in subordinate, small male lizards:
consequences for aggressiveness, color development, and parasite load. Behavioral
Ecology 8(2), 135-139. https://doi.org/10.1093/beheco/8.2.135
Salvador, A., Veiga, J.P., Civantos, E. 1999. Do skin pockets of lizards
reduce the deleterious effects of ectoparasites? An
experimental study with Psammodromus algirus. Herpetologica, 55(1),
1-7.
Schluter, D. 1984. A variance test for detecting species associations, with
some example applications. Ecology 65(3), 998-1005. https://doi.org/10.2307/1938071
Smyth, A.K., Smee, E., Godfrey, S.S., Crowther, M., Phalen, D. 2014. The
use of body condition and haematology to detect widespread threatening
processes in sleepy lizards (Tiliqua rugosa) in two agricultural
environments. Royal Society Open Science 1, 140257. https://doi.org/10.1098/rsos.140257
Soualah-Alila, H., Bouslama, Z., Bouattour, A. 2009. Parasites of lizards and
pathogens agents in Northeast of Algeria. XX Biological Science Forum and V
International Biotechnology Congress. Hammamet, Tunisia.
Soualah-Alila, H., Bouslama, Z., Amr, Z., Bani Hani, R. 2015. Tick infestations
(Acari: Ixodidae) on three lizard species from Seraidi (Annaba district),
northeastern Algeria. Experimental and Applied Acarology 67(1), 159-163.
https://doi.org/10.1007/s10493-015-9932-1
Tack, W., Madder, M., Baeten, L., Vanhellemont, M., Gruwez, R.,
Verheyen, K. 2012. Local habitat and landscape affect Ixodes ricinus tick
abundances in forests on poor, sandy soils. Forest Ecology and Management 265,
30-36. https://doi.org/10.1016/j.foreco.2011.10.028
Telford, S.R.Jr. 2008. Hemoparasites of the Reptilia: color atlas and
text. CRC Press. Boca Raton, FL. USA.
Tommassone, L., Ceballos, L.A., Ragagli, C., Martello, E., De Sousa, R.,
Stella, M.C., Mannelli, A. 2017. Importance of common wall lizards in the
transmission dynamics of tick-borne pathogens in the Northern Apennine
mountains, Italy. Microbial Ecology 74(4), 961-968. https://doi.org/10.1007/s00248-017-0994-y
Uller, T.,
Olsson, M. 2003. Prenatal exposure to testosterone
increases ectoparasite susceptibility in the common lizard (Lacerta vivipara).
Proceedings of the Royal Society of London B: Biological Sciences
270(1526), 1867-1870. https://doi.org/10.1098/rspb.2003.2451
Václav, R., Prokop, P., Fekiač, V. 2007. Expression of breeding coloration
in European green lizards (Lacerta viridis): variation with morphology
and tick infestation. Canadian Journal of Zoology 85(12), 1199-1206. https://doi.org/10.1139/Z07-102
Veiga, J.P., Salvador, A., Merino, S., Puerta, M. 1998. Reproductive effort affects immune response and parasite infection
in a lizard: a phenotypic manipulation using testosterone. Oikos 82(2),
313-318. https://doi.org/10.2307/3546971
Watkins,
H.V., Blouin-Demers, G. 2019. Body size, not age,
predicts parasite load in Clark’s Spiny Lizards (Sceloporus clarkii). Canadian
Journal of Zoology 97(3), 220-224. https://doi.org/10.1139/cjz-2017-0328
Wild, K.H.,
Gienger, C.M. 2024. Tick-tock, racing the clock:
parasitism is associated with decreased sprint performance in the Eastern fence
lizard. Biological Journal of the Linnean Society 143, blae009. https://doi.org/10.1093/biolinnean/blae009
Wu, Q., Richard, M., Rutschmann, A., Miles, D.B., Clobert, J. 2019.
Environmental variation mediates the prevalence and co-occurrence of parasites
in the common lizard, Zootaca vivipara. BMC Ecology 19, 44. https://doi.org/10.1186/s12898-019-0259-3
Zuur, A.F., Ieno, E.N., Elphick, C.S. 2010. A protocol for data
exploration to avoid common statistical problems. Methods in Ecology and
Evolution 1, 3-14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Appendix / Anexo
Table A1. Results of the models for the variation in the intensity of tick
infestation, without including the lizard with 82 ticks. Models were run
considering tick-infested lizards. The intensity of tick infestation was
log-transformed. The significant parameters (P < 0.05) are marked in
bold.
Tabla A1. Resultados
de los modelos para la variación en la intensidad de infestación por
garrapatas, sin incluir la lagartija con 82 garrapatas. Los modelos se
realizaron considerando solo las lagartijas infestadas. La intensidad de
infestación por garrapatas estuvo transformada logarítmicamente. Los parámetros
significativos (P < 0.05) están marcados en negrita.
|
Parameter
|
Type-II sum of squares
|
F-value
|
Degrees of freedom
|
P-value
|
|
Sex model
|
|
Intercept
|
53.333
|
936.353
|
1,
53
|
<
0.001
|
|
Geographic location
|
2.803
|
9.842
|
5, 53
|
< 0.001
|
|
Sex
|
0.068
|
1.185
|
1,
53
|
0.282
|
|
Life stage model
|
|
Intercept
|
54.029
|
954.714
|
1,
65
|
<
0.001
|
|
Geographic location
|
4.284
|
15.140
|
5, 65
|
< 0.001
|
|
Life stage
|
0.315
|
5.560
|
1, 65
|
0.022
|
Table A2. Results of the selected linear mixed-effects models for the
variation in the total number of leucocytes and the H/L ratio in Psammodromus
algirus lizards, without including the lizard with 82 ticks. Intensity
models were run considering tick-infested lizards. The dependent variables were
log-transformed. The significant parameters (P < 0.05) are marked in
bold.
Tabla A2. Resultados de los modelos lineales de efectos mixtos
seleccionados para examinar la variación en el número total de leucocitos y la
proporción H/L en las lagartijas, sin incluir la lagartija con 82 garrapatas.
Los modelos se realizaron considerando solo las lagartijas infestadas. La
intensidad de infestación por garrapatas estuvo transformada logarítmicamente.
Los parámetros significativos (P < 0.05) están marcados en negrita.
|
Fixed effects
|
Estimate ± se
|
t-value
|
Degrees of freedom
|
P-value
|
|
Intensity model for number of
leucocytes
(AICc) = -29.979
|
|
Intercept
|
1.642
± 0.090
|
18.166
|
16.376
|
<
0.001
|
|
Log(number of ticks)
|
-0.109
± 0.85
|
-1.292
|
43.422
|
0.203
|
|
Intensity model for H/L ratio
(AICc) = -34.110
|
|
Intercept
|
-0.220
± 0.055
|
-3.973
|
12.058
|
0.002
|
|
Sex (male)
|
-0.177 ± 0.062
|
-2.862
|
9.235
|
0.018
|
Note: in all models,
the reference category for the sex is female.
![]() , Gregorio
Moreno-Rueda2
, Gregorio
Moreno-Rueda2 ![]() , Francisco J. Zamora-Camacho3,4
, Francisco J. Zamora-Camacho3,4 ![]() , Mar Comas2,5
, Mar Comas2,5 ![]() , El-Mustapha Laghzaoui6,7
, El-Mustapha Laghzaoui6,7 ![]() , Miguel Ángel Carretero8,9,10
, Miguel Ángel Carretero8,9,10 ![]() , Afonso D. Rocha11,12
, Afonso D. Rocha11,12 ![]() , Sofía Irene Arce13
, Sofía Irene Arce13 ![]() , Emilio Civantos8,9,14
, Emilio Civantos8,9,14 ![]() , Rodrigo Megía-Palma14
, Rodrigo Megía-Palma14 ![]() , Luis P. da Silva8,9
, Luis P. da Silva8,9 ![]() , Ana Cláudia Norte15
, Ana Cláudia Norte15 ![]()

