Introduction
Alien plants can have detrimental impacts
on biodiversity, including the decline and local extinction of indigenous
species (Vilà et al. 2010; Pyšek et al. 2012a; Pérez et al. 2022). Assessing their potential impacts is important to decision making
and the application of successful management strategies (van Kleunen et al. 2018; Pyšek et al. 2020). The Poaceae family constitutes a high portion of the alien flora,
for example in Japan (372 out of 1553), China (205 out of 14710), the Czech
Republic (152 out of 1378), Italy (163 out of 1200), Belgium (118 out of 2500)
(Pyšek et al. 2012b; Galasso et al. 2018; Lin and Ma 2022; Verloove 2023) and Iran (34 out of 311) (Sohrabi et al. 2023b). Generally,
grasses possess traits that facilitate long-distance dispersal, establishment,
colonization, and transformation of novel environments, and resilience to
disturbance and drought (Linder
et al. 2017; Bastida et al. 2021; Leal et al. 2022).
Alien grasses with high seed production, long seed viability, fast seed
germination, vegetative reproduction and low palatability have high potential
to invade disturbed sites (Medeiros
and Focht 2007). They have spread over large areas
of natural ecosystems displacing native species owing to their aggressiveness
and highly competitive ability (Damasceno and Fidelis 2020).
Furthermore, alien grasses are an important threat to biodiversity conservation
due to their resilience to global warming (some are C4 species) and potential
for increasing fire risk (D’Antonio and Vitousek 1992).
One way to quantify the potential
environmental impacts of alien plants is a standard approach for ranking alien
species based on the damage they may cause in recipient areas (Nentwig et al. 2016; Blackburn et al.
2014; Vilà
et al. 2019; Kumschick et al. 2020).The IUCN
Environmental Impact Classification of Alien Taxa (EICAT-IUCN) is a scheme for
classifying alien taxa in terms of the magnitude of their environmental impacts
and the mechanism involved to prevent or limit their negative consequences (www.iucn.org).
EICAT-IUCN proposes a global assessment where the category assigned to a
species is based on the highest impact ever recorded or its highest current
impact, observed anywhere (IUCN
2020a and b; Kumschick et al. 2024).
Grasses have also large economic impacts on
agricultural lands (Milton 2004; Goggin et al. 2012; Follak and Essl
2012). For example, the rigid ryegrass (Lolium
rigidum) infestation in southeastern Australia costs an estimated $93
million (AUD) per annum in grain loss and considerably more in control costs (Bajwa et al. 2021). Johnson grass (Sorghum halepense) is classified as an
important weed in over 53 countries occurring in 30 different crops (Follak and Essl 2012; Peerzada et al.
2017), causing substantial crop yield loss (Klein and Smith 2021). There are 311 alien plants in Iran and
the number is increasing every year (Sohrabi et al. 2023b; Sohrabi and
Pagad 2024). A large proportion of alien plants in
Iran are grasses (Sohrabi et al. 2023b), but information
on their potential impact on both native species and crops is lacking. For
example, Rottboellia cochinchinensis is present in the Khozestan
province, (Dinarvnd
and Ale-Bakhit 2013; Sohrabi et al. 2023a). This species is
known worldwide for invading crops and disturbed habitats in tropical and
subtropical regions (Funez et al.
2016). However, we do not know its potential impact
in Iran and how it compares to that of other alien grasses present in the
country. Overall, there is a lack on the impact of grass invasions in Iran. To
contribute to filling this knowledge gap, the aim of this paper is to
categorize alien grasses in Iran based on their environmental impacts by using
the EICAT-IUCN classification, and furthermore, to assess potential impacts on
agricultural production. We assumed that the life cycle, photosynthetic
pathway, invasion status and distribution of the grass species in Iran´s
ecological zones contribute to the severity of the impacts. We also discuss
management options for alien grasses for both natural ecosystems and arable
lands.
Methods
The selection of plants
Thirty-four alien grasses present in Iran
were classified as casual, naturalized, and invasive based on their stage in
the invasion process (Richardson
et al. 2000; Sohrabi et al. 2023b). The potential
environmental impacts of these species were assessed following the EICAT-IUCN
guidelines, which consider 12 mechanisms of impact (IUCN 2020b; Volery et al. 2020). The severity of the
impacts was categorized as minimal concern (MC), minor (MN), moderate (MO),
major (MR), or massive (MV).
We obtained information on the
environmental and agricultural impacts of the study of alien grasses from
primary scientific literature related to their global alien range. Data on
their distribution in Iran, habitat type, introduction pathways, and
photosynthetic pathways were sourced from scientific journal articles, reports,
books, book chapters (see references in Appendix), and online databases such
as WIKTROP
(Weed Identification and Knowledge in the Tropical and
Mediterranean areas). The distribution of all alien grasses in Iran was
assigned to one of the four ecological zones of the country (Fig. 1):
(a) Hyrcanian zone: located in the north, with a temperate climate, it extends
along the southern coast of the Caspian Sea and the northern part of the
country; (b) Khalij-o-Omani zone: located in the south, covering an area of
2 130 000 ha, it is characterized by a sub-equatorial climate (Heshmati 2012);
(c) Zagross zone: situated in the west, this mountainous region covers
approximately 4 749 000 ha, characterized by a semi-arid climate and
temperate winters; and (d) Iran-o-Touranian zone: dominated by the central
Iranian plateau, this zone covers 1 648 000 km² and features a wide
variety of climates, surrounded by mountain ranges.
Statistical analysis
A contingency table was used to analyze
associations between EICAT-IUCN impact scores (harmful (MV, MR and Mo) and
non-harmful (MN, MC and DD)) and variables such as species status (casual and
naturalized invasive), life cycle (annual and perennial), and photosynthetic
pathway (C3 and C4). Fisher's exact test was applied to identify significant
relationships at 0.05 level. Statistical analyses were performed using R 4.3.0
(R Core Team 2022), and the packages ggplot2 (Wickham 2016) and ggpubr (Kassambara 2025) were used for data visualization.
Results
General characteristics of alien
grasses
In Iran, there are 22 naturalized, 11
casuals, and one invasive alien grass species. Most of
these grasses utilize the C4 photosynthetic pathway (27 species). The C4 pathway
is strongly associated with successful naturalization in new areas. Among the
22 naturalized grasses, only Five species utilize the C3 pathway. By longevity,
there is a similar number of perennial species (18) and annual species (17).
Notably, only two of the 17 annual species use the C3 photosynthetic pathway. The Poaceae family, due to their seed size,
is easily transported through different introduction
pathways (Table 1). Approximately 19 species originate from tropical and temperate
Asia, while seven are native to Africa and North America, respectively. In
Iran, contamination (e.g. seed contamination) was identified as the primary
pathway (56%) for the introduction of alien grasses. In most cases, the
introduction in Iran has been accidental (14%), with a few exceptions involving
deliberate releases, such as Bambusa vulgaris and Chrysopogon
zizanioides.
Impact assessment based on
EICAT-IUCN
Based on EICAT-IUCN framework, the most
common impact scores for alien grasses in Iran were Minor (MN) and Major (MR);
with 13 and seven species assigned to these categories, respectively. Seven
species were classified as Data Deficient (DD). Among the 22 naturalized
species, ten were rated MN, while five were categorized as MR (Table 1; Fig. 2). We
identified the species Rottboellia cochinchinensis, Paspalum
distichum, and Microstegium vimineum, as having Massive (MV)
impacts. Of these, only R. cochinchinensis is invasive in Iran, while
the others are naturalized. Additionally, Cortaderia jubata and Phyllostachys
reticulata, which are casual grasses, exhibited major impacts (Fig. 2).
Competition (23.5%) and transmission of disease (20%) were the most frequently
reported mechanisms of impact for alien grasses. Structural impacts on the
ecosystems, hybridization, and toxicity/poisoning were also common (Table 1).
Fisher's exact test revealed that there is no evidence of an association
between EICAT-IUCN scores and invasive status (p-value: 1, odds
ratio=1.03), while the association between EICAT-IUCN scores and life cycle is
considered to be statistically significant (p-value= 0.0.032, odds
ratio=0.036). Various EICAT-IUCN scores had shown insufficient evidence to
reject the null hypothesis and that there is no evidence of an association with
photosynthetic pathways (p-value = 0.74, odds ratio=0.65), although all
three grasses with MV score were C4 species.
Distribution of alien grasses in
Iran
The proportions of alien grasses in Iran's
ecological zones were 61.7% in the Hircanian, 41.1% in the Zagros, 26.4% in the
Iran-o-Turonian, and 8.8% in the Kh-O-Omanian zone. The Hircanian ecological
zone had the highest proportion of grasses with MN (47.6%) and MR (28.5%)
impact scores. In contrast, 50% of the grasses in the Zagros zone had a Minor
(MN) impact score, whereas no grasses with this score were recorded in the
Kh-O-Omanian zone. The proportion of grasses classified as Data Deficient (DD)
was higher in the Iran-o-Turonian zone than in others ecological zones (Fig. 1).
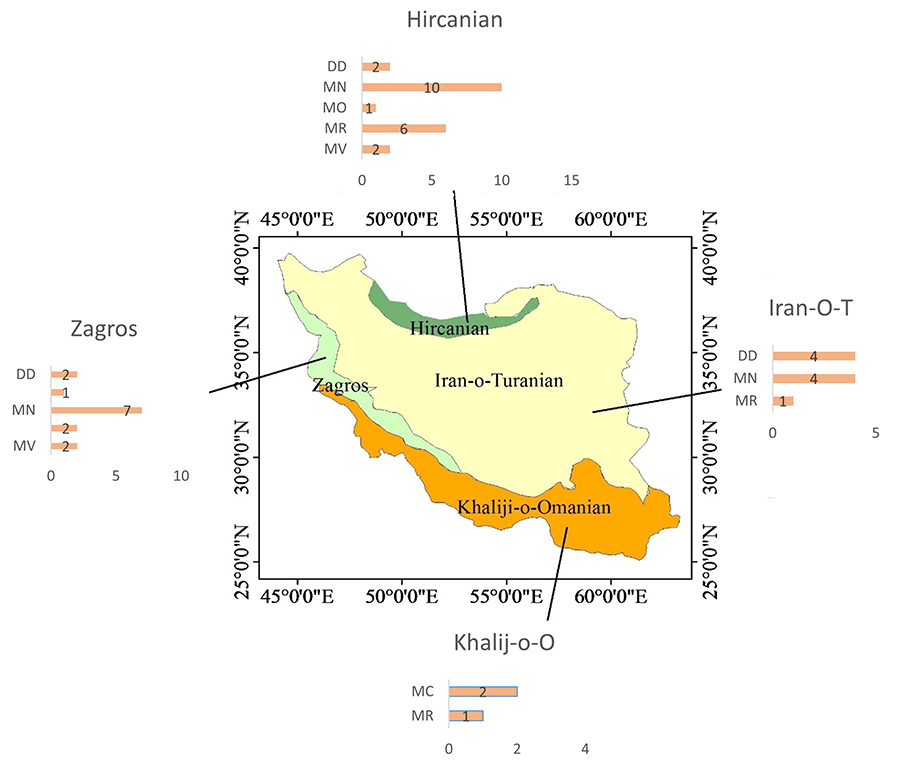
Figure 1. The distribution of 34 alien grasses in Iran along with their
EICAT-IUCN scores. The graphs display the number of species classified under
each EICAT category (DD - Data deficient, MC - Minimal concern, MN – Minor, MO
– Moderate, MR – Major and MV – Massive).
Figura 1. Distribución
de 34 gramíneas invasoras en Irán junto con sus puntuaciones EICAT-UICN. Los
gráficos muestran el número de especies clasificadas en cada categoría EICAT
(DD: datos insuficientes; MC: preocupación mínima; MN: menor; MO: moderada; MR:
mayor y MV: masiva).
Table 1. List of 34 alien grass in Iran and their main characteristics and
environmental impacts.
Tabla 1. Lista
de 34 especies de gramíneas invasoras en Irán y sus principales características
e impactos ambientales.
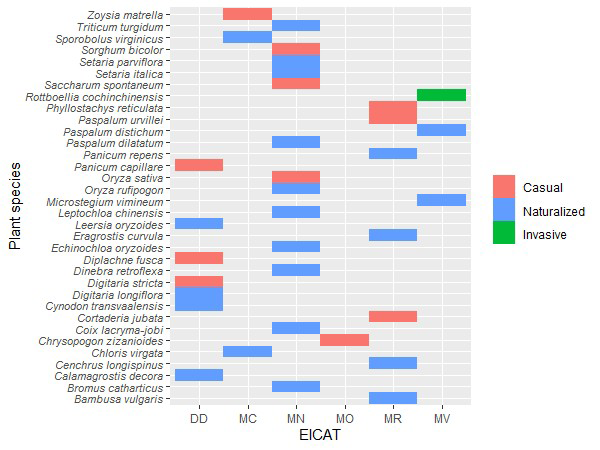
Figure 2. Invasion status and EICAT-IUCN impact score of 34 alien
grasses in Iran. EICAT-IUCN ranking included data deficiency (DD), minimal
(MC), minor (MN), moderate (MO), major (MR) or massive (MV) impact. Two cases
are subspecies: Triticum turgidum subsp. durum and Diplachne
fusca subsp. Uninervia.
Figura 2. Estado de invasión y puntuación de
impacto EICAT-UICN de 34 gramíneas invasoras en Irán. La clasificación
EICAT-UICN incluyó datos insuficientes (DD), impacto mínimo (MC), menor (MN),
moderado (MO), mayor (MR) o masivo (MV). Dos casos son subespecies: Triticum
turgidum subsp. durum y Diplachne fusca subsp. Uninervia.
Impact
of alien grasses on agricultural areas
Twenty-three of the 34 alien grass species
identified in Iran are invading agricultural areas (Table 2). The majority of these species were observed in
rice fields, comprising 14 species. Six species were observed in maize and
sugarcane fields, while only five species were recorded in orchards and
vineyards. The primary mechanisms of impact include competition with crops and
serving as hosts for pests. Furthermore, some species exhibited herbicide
resistance, allelopathic potential, and toxicity to animals (Table 2). We recorded thirteen alien weedy
grass species in northern Iran, six in the southern region, with the remaining
species distributed across central and eastern Iran (Table 2).
Table 2. Alien weedy grasses in Iran invading agricultural areas and their
characteristics. See full Literature in Appendix.
Tabla 2. Las gramíneas invasoras de zonas agrícolas en Irán y sus
características. Véase la bibliografía completa en el Apéndice.
Discussion
General characteristics of alien
grasses
Alien grasses contribute 13%, 9%, and 7.6%
to the total number of naturalized, casual, and invasive alien plants in Iran,
respectively (Sohrabi et al.
2023b). The proportion of naturalized grasses in
Iran is comparable to findings from other studies in China (Lin and Ma 2022)
and worldwide (Pertierra et
al. 2023). The faster
growth rates and larger sizes of C4 species enhance their establishment success
compared to C3 species (Jia et al.
2016). Spartina alterniflora invasion, a
perennial C4 grass in China, has been linked to its rapid growth and ability to
stabilize tidal flats (Cheng et
al. 2006). The worse invasive alien grasses have C4
photosynthetic pathway (GISD 2013). For example, Cenchrus spinifex is one of the foremost
invasive C4 grass in Hungary (Botta-Dukát and Balogh 2008). Numerous
studies have highlighted the successful establishments of the perennial grasses
from temperate-tropical regions due to their higher competitive ability in warm
climates (Hacker and
Dethier 2006; Lopes et al. 2023). In our study, most
C3 grasses were perennial. The rapid spread of these species, whether by seed
or vegetative propagation; facilitates their invasion into disturbed areas,
such as the Cerrado in Brazil (Zenni
et al. 2020). In addition, Seed contamination as the primary pathway of new introduction is
consistent with findings in Europe (Poschlod et al. 2009).
Impact assessment based on
EICAT-IUCN
Among different mechanisms competition is
well documented, while other mechanisms, such as hybridization and biofouling,
are more challenging to assess due to indirect evidence and complex data
interpretation (Foxcroft et al.
2019). For example, detecting hybridization impacts
requires molecular research, which is costly and more time-consuming compared
to evaluating allelopathy or competition (Gioria and Osborne 2014; Kalisz et al. 2021). Phenolic acids are the main identified allelochemicals (67%) in
the Poaceae family (Favaretto
et al. 2018). In addition to irritating trichomes
on the leaf sheaths of R. cochinchinensis, its phytotoxic effects
on adjacent plants specie cause reduced growth of seedlings (Meksawat and Pornprom 2010). Changes in disturbance regimes and natural succession have been
reported for grasses like Chrysopogon zizanioides, due to their root
system in upper horizons of the soil (Eab et al. 2015; Freschet and Roumet 2017; Badhon et al. 2021). The transmission of disease is another significant mechanism, and
the importance of grasses species in transmitting endophytic fungi is well
known (Lapierre and
Signoret 2004; Yuan et al. 2010). For example, Bipolaris
gigantea, a foliar fungal pathogen in alien Microstegium
populations, had significant impact on biomass responses of three native North
American grass species (Kendig et
al. 2021); and Xylella fastidiosa, a
widespread bacterial pathogen of olives, almond and citrus, can be transmitted
indirectly (xylem-feeding insects) by alien grasses (Najberek et al. 2022).
Distribution of alien grasses in
Iran
The highest number of naturalized and
invasive plants in the Hircanian ecological zone is attributed to its favorable
environmental conditions and high population density (Sohrabi et al. 2023a). Biodiversity hotspots with high environmental and economic value
are at the greatest risk of biological invasions and should therefore be
prioritized for invasive species management (Li et al. 2016; Yang et al. 2023).
The Caspian forests, located in the Hircanan zone and recognized as a UNESCO
World National Heritage site (UNESCO
2019), are a critical area in Iran that requires
attention as a potential hotspot for biological invasion. Rottboellia
cochinchinensis was found in the Zagros zone and it is the only invasive
grass species recorded in Iran. With a high seed production potential of over
3000 seeds per plant, immediate action is essential to curb its rapid spread (Dinarvnd and Ale-Bakhit 2013). Understanding the ecological zones most vulnerable to invasion is
crucial for predicting the threats posed by alien plant invasions (Sohrabi and Gherekhloo 2015). Effective management strategies can be better formulated and
implemented by combining distribution data with environmental impact scores (Panda et al. 2017; Sohrabi et al.
2023a). The agricultural lands and ruderal areas
are frequently cited as the primary habitats invaded by alien plants (Gaertner et al. 2017; Sohrabi et al.
2023b; Potgieter et al. 2024). However, it is
worth highlighting that many cultivated alien grasses have significantly
contributed to agricultural production and economic development (Randriamampianina et al. 2024). For instance, Sorghum bicolor and Cortaderia jubata
have been introduced to Iran as crops and ornamental plants, respectively.
Globally, Echinochloa oryzoides and Setaria italica are among the
most commonly encountered weedy grass species. Their frequent occurrence can be
attributed to their remarkable adaptability to various environmental stressors,
such as flooding, drought, and salinity (Mohammadvand et al. 2012; Lapuimakuni et al. 2018; Kaya-Altop et al.
2019; Nisa and Jadid 2021). Among the most
damaging species in agricultural areas are P. repens, P. distichum and L. chinensis, which pose a significant threat to crop production, potentially
causing yield losses of up to 60% (Hossain et al. 2001; Kojima et al. 2005; Hayyat et al. 2023). Paspalum distichum infestation has
been reported in perennial crops throughout Spain, where it threatens olive and
vineyard production (Costa 1997). The introduction of P. distichum for pasture purposes has
increased its potential to invade, particularly arid regions, and its
impact extends to river flora and the integrity of river systems in Portuguese
floodplains (Bernez et al. 2005; Driscoll et al.
2014). Similarly, the negative impact of P.
repens has been extensively documented in agricultural areas and native
plant communities in the USA, where its management in flood control systems for
Florida’s citrus groves alone costs $2 million annually (Cuda et al. 2007;
Langeland and Burks 2022). Leptochloa chinensis is a serious weed in the
direct-seeded rice crop, causing substantial yield reductions at high densities
due to its competitive ability, which is attributed to its C4 photosynthesis
pathway and increased biomass production rate (Benvenuti et al. 2003; Sage et al. 2012;
Deng et al. 2021). In total, there are 25 predominant weedy grass species found
across various agricultural lands in Iran, of which seven species, accounting
for 28%, are alien (Mozafarian
2021; Sohrabi et al. 2023b, 2024).
Therefore, a better understanding of the portion of alien grasses in the weed
flora and their impact can benefit and improve the ecological sustainability of
crop production systems.
Management of alien grasses
Certain interventions can reduce the
propagule pressure and the likelihood of establishment and spread of newly
introduced alien grasses (Perrings
et al. 2005; Simberloff 2009; Siddiqui et al. 2022). Strategies such as limiting contamination vectors, monitoring
pathways for target grasses, controlling contaminants, and adopting long-term
approaches have been suggested as the most effective methods for managing
invasive grasses in specific areas (Pyšek et al. 2011; Gentili et al. 2021; Rudell et al. 2023). However, we highlight that the most accurate management options
will depend on the infested habitat (natural areas or agricultural lands) and
the life cycle of the species (annual or perennial). In natural areas, the most
effective control methods often involve an integrated approach that include
burning, mowing, hand clearing, and herbicides application (Marushia and Allen 2011). In agricultural areas, effective control typically involves the
use of certified seeds, proper land preparation, stale seedbeds to promote weed
germination prior to seeding, careful crop and water management, herbicides,
and crop rotation (Milton 2004; Zand et al. 2017; Khasraw et al. 2023; Gherekhloo et
al. 2020; Siddiqui et al. 2022).
The application of herbicides is a widely
used strategy for managing invasive plant species, particularly in agricultural
systems. However, the continuous and intensive use of these chemicals has
resulted in the emergence of herbicide-resistant weed populations, posing
significant challenges to crop productivity and sustainability (Hassanpour-bourkheili et al.
2024; Heap
2024). To achieve complete control of P. repens
in naturally infested fields often requires multiple applications of methyl
sulfanilylcarbamate (a dihydropteroate synthase inhibitor), as plowing-induced
rhizome fragmentation disperses the weed across various soil layers,
complicating eradication (Hossain
2001). In paddy fields in Rasht, Iran,
bensulfuron-methyl, an acetolactate synthase inhibitor, has demonstrated higher
efficacy in controlling Echinochloa oryzoides (Pouramir and Yaghoubi 2020). In USA rice fields, Leptochloa chinensis and P.
distichum have evolved herbicide resistance due to the repeated application
of a single herbicide or herbicides group (Chauhan et al. 2012). To manage P.
distichum and mitigate the risk of glyphosate resistance, the integration
of herbicides with different modes of action has been recommended (Alcantara et al. 2016).
As a sustainable alternative to herbicides,
biological control methods have been suggested, including the use of grazing
animals such as rabbits, goats, sheep, and ducks, as well as cover crops and
intercropping. These approaches have been explored for managing invasive
species like Paspalum repens, P. distichum, and Rottboellia
exaltata in turmeric fields (Prabhakaran Nair 2013).
Relying on a single control tactic may be
insufficient for effectively managing invasive plant species, particularly
given the high reproductive potential and dispersal ability of certain species.
In this context, Integrated Weed Management (IWM) presents a promising strategy
for controlling and mitigating the impact of invasive species in both
agricultural and natural ecosystems (Gonzalez-Andujar 2023).
Conclusion
This study, based on the EICAT-IUCN
assessment, highlights the significant impact potential of several alien grass
species in Iran, including Rottboellia cochinchinensis, Paspalum
distichum and Microstegium vimineum. These species primarily affect
ecosystems through competition, disease transmission, and structural
alterations. The prioritization of management efforts should be guided by these
findings, particularly for P. distichum and Panicum repens which
pose substantial threats to biodiversity and agricultural productivity. The
highest number and impacts of alien grasses in Iran were found in the Hircanian
zone, followed by the Iran-o-Turanian zone.
Notably, a correlation between EICAT-IUCN
score and life cycle underscores the critical role of perennial and species in
driving environmental impact. Given the prevailing warming conditions, it
is crucial to focus on the introduction and management of alien C4 perennial
grasses in Iran. Effective management of strategies will depend on the life
cycle of the species and the nature of the invaded habitat, though the most
successful approaches typically involve a combination of methods.
Data Availability
More information
is available in Appendix and raw data will be presented upon reasonable
request to the corresponding author.
Financing, required permits, potential
conflicts of interest and acknowledgments
Gorgan University of Agricultural Sciences
and Natural Resources (GUASNR), Iran supported this research (project no.
GAU-03-508-20). We would like to thank Sabrina Kumschick for her valuable
suggestions. The authors are grateful to anonymous reviewers for their valuable
comments that greatly enhanced this work.
The authors
declare that they have no conflicts of interest.
Authors' contribution
SS, JG, JLG-A, MV, designed the study; SS
collected the data; SS analyzed the data; SS led the writing of the manuscript,
and all authors contributed critically to the drafts and gave final approval
for publication.
References
Alcantara, R., Fernández-Moreno,
P., Smeda, R.J., Alves, P.L., De Prado, R. 2016. Response of Eleusine
indica and Paspalum distichum to glyphosate following repeated use
in citrus groves. Crop Protection 79:1–7. https://doi.org/10.1016/j.cropro.2015.09.027
Badhon, F.F., Islam M.S., Islam M.A. 2021. Contribution of Vetiver root on
the improvement of slope stability. Indian Geotechnical Journal 51:
829–840. https://doi.org/10.1007/s40098-021-00557-0
Bajwa, A.A., Latif, S., Borger, C., Iqbal, N., Asaduzzaman, M., Wu, H.,
Walsh, M. 2021. The Remarkable Journey of a Weed: Biology and Management of
Annual Ryegrass (Lolium rigidum) in Conservation Cropping Systems of
Australia. Plants 10: 1505. https://doi.org/10.3390/plants10081505
Bastida, F., Menendez, J.,
Camacho, D., Gonzalez-Andujar, J.L. 2021. Season-long seed
dispersal patterns of the invasive weed Erigeron bonariensis in
south-western Spain. Crop Protection 148: 105720. https://doi.org/10.1016/j.cropro.2021.105720
Benvenuti, S., Dinelli, G., Bonetti, A. 2004. Germination ecology of Leptochloa
chinensis: A new weed in the Italian rice agro-environment. Weed
Research 44 (2): 87-96. https://doi.org/10.1111/j.1365-3180.2003.00376
Bernez, I., Ferreira, M.T., Albuquerque, A., Aguiar, F. 2005. Relations Between River Plant Richness in the Portuguese Floodplains
and the Widespread Water Knotgrass (Paspalum Paspalodes). Hydrobiologia
551: 121–130. https://doi.org/10.1007/s10750-005-4454-1
Blackburn, T.M., Essl, F., Evans, T., Hulme, P.E., Jeschke, J.M., Kühn, I.,
et al. 2014. A Unified Classification of Alien Species Based on the Magnitude
of their Environmental Impacts. PLoS Biology 12(5): e1001850. https://doi.org/10.1371/journal.pbio.1001850
Botta-Dukát, Z., Balogh,
L. 2008. The most important invasive plants in Hungary. HAS Institute of Ecology and Botany, Vácrátót, Hungary. 255 pp.
Chauhan, B.S., Mahajan, G., Sardana, V., Timsina, J., Mangi, L.J. 2012.
Productivity and Sustainability of the Rice–Wheat Cropping System in the
Indo-Gangetic Plains of the Indian subcontinent: Problems, Opportunities, and
Strategies. In: Donald L. Sparks (ed.), Advances in Agronomy, Chapter
Six, 117: 315-369, Academic Press. https://doi.org/10.1016/B978-0-12-394278-4.00006-4
Cheng, X., Luo, Y., Chen, J., Lin, G., Chen, J., Li, B. 2006. Short-term
C4 plant Spartina alterniflora invasions change the soil carbon in C3
plant-dominated tidal wetlands on a growing estuarine Island, Soil Biology
and Biochemistry 38(12): 3380-3386, https://doi.org/10.1016/j.soilbio.2006.05.016
Costa, J. 1997. From research to practice: staying ahead of the problem. In: De
Prado, R., Jorrin, J. and Garcia-Torres, L. (eds.), Weed and Crop Resistance
to Herbicides, pp. 315-320. Kluwer Academic Publishers, Dordrecht, The
Netherlands. https://doi.org/10.1007/978-94-011-5538-0_35
Cuda, J. P., Dunford, J.C., Leavengood, J.M. 2007. Invertebrate Fauna
Associated with Torpedograss, Panicum repens (Cyperales: Poaceae), in
Lake Okeechobee, Florida, and Prospects for Biological Control. Florida Entomologist 90(1): 238-248. https://doi.org/10.1653/0015-4040(2007)90[238:IFAWTP]2.0.CO;2
D’Antonio,
C.M., Vitousek, P.M. 1992. Biological Invasions by
Exotic Grasses, the Grass/Fire Cycle, and Global Change. Annual Review of
Ecology and Systematics 23: 63–87. https://doi.org/10.1146/annurev.es.23.110192.000431
Damasceno,
G., Fidelis, A. 2020. Abundance
of invasive grasses is dependent on fire regime and climatic conditions in
tropical savannas. Journal of Environmental Management 271: 111016, http://dx.doi.org/10.1016/j.jenvman.2020.111016
Deng, W., Yang, M., Li, Y., Xia, Z., Chen, Y., Yuan, S., Yang, Q. 2021.
Enhanced metabolism confers a high level of cyhalofop-butyl resistance in a
Chinese sprangletop (Leptochloa chinensis (L.) Nees) population. Pest
Management Science. 77(5):2576-2583. https://doi.org/10.1002/ps.6297.
Epub 2021 Feb 12. PMID: 33497007.
Dinarvand,
M., Ale-Bakhit, M. 2013. Rottboellia chinensis,
a new weed for Iran. Rostaniha 14(2): 246-247.
Driscoll, D.A., Catford, J.A., Barney, J.N., Hulme, P.E., Inderjit, Martin
T.G., Pauchard, A., et al. 2014. New pasture plants intensify invasive species
risk. Proceedings of the National Academy of Sciences USA, 111, 16622-7.
https://doi.org/10.1073/pnas.1409347111
Eab, K.H., Likitlersuang, S., Takahashi, A. 2015. Laboratory and
modelling investigation of root-reinforced system for slope stabilization. Soils
Found 55(5): 1270–1281. https://doi.org/10.1016/j.sandf.2015.09.025
Favaretto, A., Scheffer-Basso, S.M., Perez, N.B. 2018. Allelopathy in Poaceae
species present in Brazil. A review. Agronomy for Sustainable Development 38:
22. https://doi.org/10.1007/s13593-018-0495-5
Follak, S., Essl, F. 2012. Spread dynamics and agricultural impact of Sorghum
halepense, an emerging invasive species in Central Europe. Weed Research
53(1): 53-60. https://doi.org/10.1111/j.1365-3180.2012.00952.x
Foxcroft, L.C., Spear, D., van Wilgen, N.J., McGeoch, M.A. 2019. Assessing
the association between pathways of alien plant invaders and their impacts in
protected areas. NeoBiota 43: 1-25. https://doi.org/10.3897/neobiota.43.29644
Freschet,
G.T., Roumet, C. 2017. Sampling roots to capture
plant and soil functions. Functional Ecology 31(8): 1506–1518. https://doi.org/10.1111/1365-2435.12883
Funez, L.A., Ferreira, J.P., Hassemer, G., Trevisan, R. 2016. First
record of the invasive species Rottboellia cochinchinensis (Poaceae,
Andropogoneae) in the South Region of Brazil. Check List 12(4): 1-4. https://doi.org/10.15560/16841
Gaertner, M., Wilson, J.R.U., Cadotte, M.W., MacIvor, J.S., Zenni, R.D.,
Richardson, D.M. 2017. Non-native species in urban environments: patterns,
processes, impacts and challenges. Biological Invasions 19: 3461–3469. https://doi.org/10.1007/s10530-017-1598-7
Galasso, G., Conti, F., Peruzzi, L., Ardenghi, N. M. G., Banfi, E.,
Celesti-Grapow, L., Bartolucci, F. 2018. An updated checklist of the vascular
flora alien to Italy. Plant Biosystems - An International Journal Dealing with
All Aspects of Plant Biology, 152(3), 556–592. https://doi.org/10.1080/11263504.2018.1441197
Gentili, R., Schaffner, U., Martinoli, A., Citterio, S. 2021. Invasive
alien species and biodiversity: Impacts and management. Biodiversity
22(1-2): 1– 3. https://doi.org/10.1080/14888386.2021.1929484
Gherekhloo, J., Alcantara-de la
Cruz, R., Osuna, M. D., Sohrabi, S., Prado, R. 2020. Assessing
Genetic Variation and Spread of Phalaris minor Resistant to ACCase
Inhibiting Herbicides in Iran. Planta Daninha 38: e020195618. https://doi.org/10.1590/S0100-83582020380100026
Gioria, M.,
Osborne, B.A. 2014. Resource
competition in plant invasions: emerging patterns and research needs. Frontiers
in Plant Science 5: 501. https://doi.org/10.3389/fpls.2014.00501
GISD. 2013. Global invasive species database. 100 of the World's Worst
Invasive Alien Species. https://www.iucngisd.org/gisd/100_worst.php [Accessed on
18-02-2025].
Goggin, D.E., Powles, S.B., Steadman, K.J. 2012. Understanding Lolium
rigidum Seeds: The Key to Managing a Problem Weed? Agronomy 2(4):
222-239. https://doi.org/10.3390/agronomy2030222
Gonzalez-Andujar, J.L. 2023. Integrated Weed Management: a shift towards more sustainable
and holistic practices. Agronomy 13(10): 2646. https://doi.org/10.3390/agronomy13102646
Hacker, S.D.,
Dethier, M.N. 2006. Community modification by a
grass invader has differing impacts for marine habitats. Oikos 113(2):
279 -286. https://doi.org/10.1111/j.2006.0030-1299.14436.x
Hassanpour-bourkheili, S., Gherekhloo, J., Soltani, A., Haghnama, K., Sohrabi, S., Ziaee,
F., Taheri, M., et al. 2024. Impact of herbicide resistance on energy use and
greenhouse gas emission in wheat fields: A case study in Golestan province,
Iran. Advances in Weed Science 42: e020240016. https://doi.org/10.51694/AdvWeedSci/2024;42:00014
Hayyat, M., Safdar, M., Javaid, M., Ullah, S., Chauhan, B. 2023.
Estimation of the economic threshold of Leptochloa chinensis (Chinese
sprangletop) in direct-seeded fine grain rice (Oryza sativa). Semina: Ciências Agrárias 44(2):
803-822. https://doi.org/10.5433/1679-0359.2023v44n2p803
Heap, I. 2024. The International Herbicide-Resistant Weed Database. https://www.weedscience.org/Home.aspx. [Accessed on 18-02-2025].
Heshmati, G. 2012. Vegetation characteristics of four ecological zones of
Iran. International Journal of Plant Production 1(2): 215-224. https://doi.org/10.22069/ijpp.2012.538
Hossain, M.A., Kuramochi, H., Ishimine, Y., Akamine, H. 2001. Application
timing of asulam for torpedograss (Panicum repens L.) control in
sugarcane in Okinawa Island. Weed Biology and Management 1(2): 108-114. https://doi.org/10.1046/j.1445-6664.2001.00021.x
IUCN. 2020a. IUCN EICAT Categories and Criteria. The
Environmental Impact Classification for Alien Taxa (First edition), IUCN. X
+ Xpp. Gland, Switzerland and Cambridge, UK. https://portals.iucn.org/library/sites/library/files/documents/2020-026-En.pdf
IUCN. 2020b. Guidelines for using the IUCN Environmental Impact
Classification for Alien Taxa (EICAT) Categories and Criteria.
Version 1.1. IUCN. Gland, Switzerland and Cambridge, UK. https://iucn.org/sites/default/files/2023-02/eicat-guidelines-final-v1.1.pdf
Jia, J., Dai, Z., Li, F., Liu, Y. 2016. How Will Global Environmental
Changes Affect the Growth of Alien Plants? Frontiers in Plant Science
1(7): 1623. https://doi.org/10.3389/fpls.2016.01623
PMID: 27847511; PMCID: PMC5088532.
Kalisz, S., Kivlin, S.N., Bialic-Murphy, L. 2021. Allelopathy is pervasive
in invasive plants. Biological Invasions 23: 367–371. https://doi.org/10.1007/s10530-020-02383-6
Kassambara, A. 2025. ggpubr: 'ggplot2' Based Publication Ready Plots. R
package version 0.6.1, https://rpkgs.datanovia.com/ggpubr/
Kaya-Altop, E., Şahin, M., Jabran, K., Phillippo, C.J., Zandstra, B.H.,
Mennan, H. 2019. Effect of different water management strategies on competitive
ability of semi-dwarf rice cultivars with Echinochloa oryzoides. Crop
Protection 116: 33-42. https://doi.org/10.1016/j.cropro.2018.10.009
Kendig, A.E., Svahnström, V.J., Adhikari, A., Harmon, P.F., Flory, S.L.
2021. Emerging fungal pathogen of an invasive grass: Implications for
competition with native plant species. PLoS One 1:16(3): e0237894. https://doi.org/10.1371/journal.pone.0237894
PMID: 33647021; PMCID: PMC7920361.
Khasraw, M.N., Kareem, S.H.S., Mustafa, K.M., Aziz, O.K.K., Arif, M.,
Anwar, A., Hussain, M. 2023. Integrated weed management in wheat by using
sowing time, seed rate and herbicides. Advances in Weed Science 41:
e020230037. https://doi.org/10.51694/AdvWeedSci/2023;41:00022
Klein, P., Smith, C.M. 2021. Invasive Johnsongrass, a threat to native grasslands
and agriculture. Biologia 76: 413–420. https://doi.org/10.2478/s11756-020-00625-5
Kojima, K., Kawana, Y., Ushiki, J. 2005. Growth of two perennial grass
weeds (Paspalum distichum L. and Leersia japonica Makino) under
different weed control levels in rice. Journal of Weed Science and
Technology 50: 104-105. https://doi.org/10.3719/weed.50.Supplement_104
Kumschick, S., Bacher, S., Bertolino, S., Blackburn, T.M., Evans, T., Roy,
H.E., Smith, K. 2020. Appropriate uses of EICAT protocol, data and
classifications. NeoBiota 62: 193-212. https://doi.org/10.3897/neobiota.62.51574
Kumschick, S., Bertolino, S., Blackburn, T.M., Brundu, G., Costello, K.E., de
Groot, M., Evans, T., et al. 2024. Using the IUCN Environmental Impact
Classification for Alien Taxa to inform decision-making. Conservation
Biology 38(2): e14214. https://doi.org/10.1111/cobi.14214. Epub 2023 Dec 5.
PMID: 38051018. https://doi.org/10.1111/cobi.14214
Langeland,
K.A., Burks, K.C. 2022. Identification and
Biology of Non-native Plants in Florida's Natural Areas, Second Ed. Pub.
No. 257. Institute of Food and Agricultural Sciences. University of Florida. Gainesville,
FL, USA. http://ifasbooks.ifas.ufl.edu/p-197-identification-and-biology-of-non-native-plants-in-floridas-natural-areas.aspx
Lapierre,
H., Signoret, P.A. 2004. Viruses and Virus Diseases of Poaceae (Gramineae). Editions INRA, Paris, France. 857 p. Available at: https://www.quae.com/extract/2218
Lapuimakuni, S., Khumaida, N.,
Ardie, S.W. 2018. Short Communication: Evaluation of drought
tolerance indices for genotype selection of foxtail millet (Setaria italica).
Tropical Drylands 2(2): 37–40. https://doi.org/10.13057/tropdrylands/t020201
Leal, R.P., Silveira, M.J., Petsch, D.K., Mormul, R.P., Thomaz, S.M.
2022. The success of an invasive Poaceae explained by drought resilience but
not by higher competitive ability. Environmental and Experimental Botany
194(4): 104717. https://doi.org/10.1016/j.envexpbot.2021.104717
Li, X., Liu, X., Kraus, F., Tingley, R., Yiming, L. 2016. Risk of
biological invasions is concentrated in biodiversity hotspots. Frontiers in
Ecology and the Environment 14(8): 411-417. https://doi.org/10.1002/fee.1321
Lin, Q.W., Ma, J.S. 2022. Catalogue of Alien Plants in China.
V1: Science Data Bank, 2022. China Forestry Publishing House. Beijing, China.
[Accessed on 2024-10-08]. https://doi.org/10.57760/sciencedb.01711
Linder, H.P., Lehmann CLehmann, C.E.R., Archibald, S., Osborne, C.P.,
Richardson, D.M. 2017. Global grass (Poaceae) success underpinned by traits
facilitating colonization, persistence and habitat transformation. Biological
Reviews 93(2): 1125–1144. https://doi.org/10.1111/brv.12388
Lopes, A., O. Demarchi, L., Piedade, M.T., Schöngart, J., Wittmann, F.,
Munhoz, C., Ferreira, C., et al. 2023. Predicting the range expansion of
invasive alien grasses under climate change in the Neotropics. Perspectives
in Ecology and Conservation 21 (2), 128-135. https://doi.org/10.1016/j.pecon.2023.02.005
Marushia,
R.G., Allen, E.B. 2011. Control of Exotic Annual
Grasses to Restore Native Forbs in Abandoned Agricultural Land. Restoration Ecology 19: 45 - 54. https://doi.org/10.1111/j.1526-100X.2009.00540.x
Medeiros,
R.B., Focht, T. 2007. Invas~ao, prevenc~ao,controle
e utilizac~ao do capim-annoni-2 (Eragrostis plana Nees) no Rio Grande do
Sul, Brasil. Pesquisa Agropecuaria Gaucha 13(1/2): 105–14.
Meksawat,
S., Pornprom, T. 2010. Allelopathic effect of
itchgrass (Rottboellia cochinchinensis) on seed germination and plant
growth. Weed Biology and Management 10(1): 16–24. https://doi.org/10.1111/j.1445-6664.2010.00362.x
Milton, S.J. 2004. Grasses as invasive alien plants in South Africa. South
African Journal of Science 100: 69-75.
Mohammadvand, E., Koocheki, A., Nassiri Mahallati, M., Shahdi, A. 2012. The
effects of seed burial and flooding depths on the emergence and seedling growth
of watergrass (Echinochloa oryzoides) and barnyardgrass (E.
crus-galli). Iranian Journal of Field Crop Research 10(4): 699-708.
(In persian)
Mozafarian, V. 2021. Identification of weeds in Iran. Shahre Ab press,
Iran.1001.
Najberek, K., Olszańska, A., Tokarska-Guzik, B., Mazurska, K., Dajdok, Z.,
Solarz, W. 2022. Invasive alien species as reservoirs for pathogens. Ecological
Indicators 139: 108879, https://doi.org/10.1016/j.ecolind.2022.108879
Nentwig, W., Bacher, S., Pyšek, P., Vilà, M., Kumschick, S. 2016. The
Generic Impact Scoring System (GISS): A standardized tool to quantify the
impacts of alien species. Environmental Monitoring and Assessment 188:
315. https://doi.org/10.1371/journal.pbio.1001850
Nisa, C., Jadid, N. 2021. Exogenous acetic acid pre-treatment increases drought
tolerance of two Indonesian foxtail millet (Setaria italica) accessions.
Biodiversitas 22(4): 2117–2124. https://doi.org/10.13057/biodiv/d220460
Panda, R., Behera, M., Roy, P. 2017.
Assessing distributions of two invasive species of contrasting
habits in future climate. Journal of Environmental Management 213:
478-488. https://doi.org/10.1016/j.jenvman.2017.12.053
Peerzada, A.M., Ali, H.H., Hanif, Z., Ahsan Bajwa, A., Kebaso, L., Frimpong,
D., Iqbal, N., et al. 2017. Eco-biology, impact, and management of Sorghum
halepense (L.) Pers. Biological Invasions 25(4): 955–973. https://doi.org/10.1007/s10530-017-1410-8
Pérez, G., Vilà, M., Gallardo, B. 2022. Potential
impact of four invasive alien plants on the provision of ecosystem services in
Europe under present and future climatic scenarios. Ecosystem
Services 56, 101459. https://doi.org/10.1016/j.ecoser.2022.101459
Pertierra, L.R., Martínez, P.A.,
Rubalcaba, J.G., et al. 2023. Contrasting patterns in
phylogenetic and biogeographic factories of invasive grasses (Poaceae) across
the globe. npj biodiversity 2, 11. https://doi.org/10.1038/s44185-023-00016-4
Perrings, C., Dehnen-Schmutz, K., Touza, J., Williamson, M. 2005. How to
manage biological invasions under globalization. Trends in Ecology &
Evolution 20(5): 212–215. https://doi.org/10.1016/j.tree.2005.02.011
Poschlod, P., Baumann, A., Karlík, P. 2009. Origin and development of
grasslands in central Europe. In: Veen P, Jefferson R, de Smidt J, Van der
Straaten J. (eds), Grasslands in Europe of high nature value, pp. 15–25,
KNNV Publishing, Zeist, The Netherlands. https://doi.org/10.1163/9789004278103_003
Potgieter, L.J., Li, D., Baiser, B., Kühn, I., Aronson, M.F.J., Carboni, M.,
Celesti-Grapow, L., et al. 2024. Cities Shape the Diversity and Spread of
Nonnative Species. Annual review of ecology, evolution, and systematics
02. https://doi.org/10.1146/annurev-ecolsys-102722-012749
Pouramir,
F., Yaghoubi, B. 2021. 'Biology and management of
the invasive (Echinochloa oryzoides (Ard.) Fritsch) and common (Echinochloa
crus-galli (L.) Beauv.) barnyardgrass in paddy field. Iranian Journal of
Weed Science 17(1): 71-84. https://doi.org/10.22092/ijws.2020.128351.1357
Prabhakaran Nair, K.P. 2013. The Agronomy of Turmeric, In:
Prabhakaran Nair K.P. (ed.), The Agronomy and Economy of Turmeric and
Ginger. The Invaluable Medicinal Spice Crops, pp. 79-96 (Chapter 6),
Elsevier. https://doi.org/10.1016/B978-0-12-394801-4.00006-5
Pyšek, P., Jarosık, V., Pergl, J. 2011. Alien Plants Introduced by
Different Pathways Differ in Invasion Success: Unintentional Introductions as a
Threat to Natural Areas. PLoS ONE 6: e24890. https://doi.org/10.1371/journal.pone.0024890
Pyšek, P., Danihelka, J., Sádlo, J., Chrtek, J.J., Chytrý, M., Jarošík,
V., Kaplan, Z., et al. 2012a. Catalogue of alien plants of the Czech Republic
(2nd edition): checklist update, taxonomic diversity and invasion patterns. Preslia
94(4): 155–255.
Pyšek, P., Jarosik, V., Hulme, P., Pergl, J., Hejda, M., Schaffner, U.,
Vilà, M. 2012b. A global assessment of invasive plant impacts on resident
species, communities and ecosystems: the interaction of impact measures,
invading species’ traits and environment. Global Change Biology 18(5):
1725–37. https://doi.org/10.1111/j.1365-2486.2011.02636.x
Pyšek, P., Hulme, P.E., Simberloff, D., Bacher, S., Blackburn, T.M.,
Carlton, J.T., Dawson, W., et al. 2020. Scientists’ warning on invasive alien
species. Biological Reviews 95(6): 1511–1534. https://doi.org/10.1111/brv.12627
R Core Team. 2022. R: A language and environment for statistical computing,
Version (4.3.0). R Foundation for Statistical Computing, https://www.R-project.org
Randrianarimanana, N.F.H., Rakotomalala, N.H., MacKinnon, L., Rakotoarinivo, M.,
Randriamampianina, J.-A., Ralimanana, H., Ryan, P., et al. 2024. Local
perceptions of the benefits versus negative impacts of weedy grasses in central
Madagascar, with a focus on the genus Digitaria. Plants People Planet
6(3): 710–728. https://doi.org/10.1002/ppp3.10495
Richardson, D.M., Pyšek, P., Rejmánek, M., Barbour, M.G., Panetta, F.D., West,
C.J. 2000. Naturalization and invasion of alien plants: concepts and
definitions. Diversity and Distributions 6: 93-107. https://doi.org/10.1046/j.1472-4642.2000.00083.x
Rudell, E.C., Zanrosso, B.A., Frandaloso, D., Giacomini, A.J., Spadotto,
D.V., Vargas, L., Nunes, A.L., et al. 2023. Integrated weed management
strategies in a long-term crop rotation system. Advances in Weed Science 41:
e020220053. https://doi.org/10.51694/AdvWeedSci/2023;41:00026
Siddiqui, A.O., Yazlık, A., Jabran, K. 2022. Weed Management and Climate
Change. In: Jatoi, W.N., Mubeen, M., Ahmad, A., Cheema, M.A., Lin, Z., Hashmi,
M.Z. (eds) Building Climate Resilience in Agriculture. Springer, Cham. https://doi.org/10.1007/978-3-030-79408-8_14
Sage, R.F., Sage, T.L., Kocacinar, F. 2012. Photorespiration and the
Evolution of C4 Photosynthesis. Annual Review of Plant Biology 63: 19–47.
https://doi.org/10.1146/annurev-arplant-042811-105511
Simberloff, D. 2009. The role of propagule pressure in biological invasions.
Annual Review of Ecology and Systematics 40: 81–102. https://doi.org/10.1146/annurev.ecolsys.110308.120304
Sohrabi, S., Gherekhloo, J. 2015. Investigating status of the invasive
weeds of Iran. Proceeding of 6th Iranian Weed Science Congress. 1-3
September, Birjand, Iran. (In Persian).
Sohrabi, S., Pagad, S. 2024. Global Register of Introduced and Invasive
Species - Iran. Version 1.3. Invasive Species Specialist Group ISSG.
Checklist dataset. https://cloud.gbif.org/griis/resource?r=griis-iran&v=1.3 [Accessed on 18-02-2025].
Sohrabi, S., Gherekhloo, J., Zand, E., Nezamabadi, N. 2023a. The necessity
of monitoring and assessing alien plants in Iran. Iran Nature 8(1):
81-90. (In Persian).
Sohrabi, S., Naqinezhad, A., Kortz, A., Hejda, M., Gherekhloo, J., Zand,
E., Pergl, J., Brundu, G., Pyšek, P. 2023b. Alien flora of Iran: Species
status, introduction dynamics, habitats and pathways. Biological Invasions
25(5): 1359–1371. https://doi.org/10.1007/s10530-023-03001-x
Sohrabi, S., Gherekhloo, J., Hassanpour-bourkheili, S., Soltani, A.,
Gonzalez-Andujar, J.L. 2024. Factors Influencing the Variation of Plants’
Cardinal Temperature: A Case Study in Iran. Plants 13(20): 2848. https://doi.org/10.3390/plants13202848
UNESCO 2019. Hyrcanian Forests. World Heritage List. https://whc.unesco.org/en/list/1584/
[Accessed 18 Febr. 2025].
van Kleunen, M., Essl, F., Pergl, J., Brundu, G., Carboni, M., Dullinger, S.,
Early, R., et al. 2018. The changing role of ornamental horticulture in alien
plant invasions. Biological Reviews 93: 1421–1437. https://doi.org/10.1111/brv.12402
Verloove, F. 2023. [Poaceae family]. Manual of the Alien Plants of
Belgium. Botanic Garden Meise, Belgium. https://alienplantsbelgium.myspecies.info/
[Accessed 2/6/2023]
Vila, M., Basnou, C., Pyšek, P., Josefsson, M., Genovesi, P., Gollasch,
S., Nentwig, W., et al. 2010. How well do we understand the impacts of alien
species on ecosystem services? A pan-European, cross-taxa assessment. Frontiers
in Ecology and the Environment 8: 135–44. https://doi.org/10.1890/080083
Vilà, M., Gallardo, B., Preda, C., García-Berthou,
E., Essl, F., Kenis, M., Roy, H.E., et al. 2019. A review of
impact assessment protocols of non-native plants. Biological Invasions 21(3):
709-723. https://doi.org/10.1007/s10530-018-1872-3
Volery, L., Blackburn, T.M., Bertolino, S., Evans, T., Genovesi, P.,
Kumschick, S., Roy, H.E., Smith, K.G., Bacher, S. 2020. Improving the
Environmental Impact Classification for Alien Taxa (EICAT): a summary of
revisions to the framework and guidelines. NeoBiota 62: 547–567. https://doi.org/10.3897/neobiota.62.52723
Wickham, H. 2016. ggplot2: Elegant Graphics for Data Analysis.
Springer-Verlag New York. ISBN 978-3-319-24277-4, https://ggplot2.tidyverse.org
Yang, Y., Bian, Z., Ren, W., Wu, J., Liu, J., Shrestha, N. 2023. Spatial patterns and hotspots of plant invasion in China. Global
Ecology and Conservation 43: e02424. https://doi.org/10.1016/j.gecco.2023.e02424
Yuan, Z.L., Zhang, C., Lin, F.C. 2010. Role of diverse non-systemic
fungal endophytes in plant performance and response to stress: Progress and
approaches. Journal of Plant Growth Regulation 29(1): 116–126. https://doi.org/10.1007/s00344-009-9112-9
Zand, E., Baghestani, M.A., Nezamabadi, N., Shimi, P., Mousavi, S.K.
2017. A guide to chemical control of weeds in Iran. Iranian Research
Institute of Plant Protection. Tehran, Iran, 223 pp.
Zenni, R.D., Cunha, W.L., Musso, C., Souza, J.V., Nardoto, G.B.,
Miranda, H.S. 2020. Synergistic impacts of co-occurring invasive grasses cause
persistent effects in the soil-plant system after selective removal.
Functional Ecology 34:1102–1112, http://dx.doi.org/10.1111/1365-2435.13524
Appendix / Anexo
Literature
on the environmental and agricultural impacts of alien grasses species in Iran
cited on Table 2.
Bibliografía
sobre los impactos medioambientales y agrícolas de las especies de gramíneas
invasoras en Irán citadas en la Tabla 2.
Bromus
catharticus Vahl
Aulicino, M.B., Arturi, M.J. 2002.
Phenotypic diversity in Argentinean populations of Bromus catharticus
(Poaceae). Genetic and environmental components of quantitative traits. New
Zealand Journal of Botany 40, 223–234. https://doi.org/10.1080/0028825X.2002.9512785
Dastgheib, F., Rolston, M., Archie, W.
2003. Chemical control of brome grasses (Bromus spp.) in cereals. New
Zealand Plant Protection 56, 227–232. https://doi.org/10.30843/nzpp.2003.56.6096
Hamzeh'ee, B., Alemi, M., Attar, F.,
Ghahreman, A. 2007. Bromus catharticus and Bromus danthoniae var.
uniaristatus (Poaceae), two new records from Iran. The Iranian
Journal of Botany 13(1), pp. 33-36.
Kloppers, F.J., Pretorius Z.A. 1993. Bromus
catharticus: A new host record for wheat stem rust in South Africa. Plant
Disease 77: 1063. https://doi.org/10.1094/PD-77-1063B
Poggio, S.L., Satorre, E.H., de la Fuente, E.B. 2004. Structure of weed communities occurring in pea and wheat crops in
the rolling Pampa (Argentina). Agriculture, Ecosystems &
Environment 103, 225–235. https://doi.org/10.1016/j.agee.2003.09.015
Yanniccari, M., Vázquez-García, J.G., Gómez-Lobato, M.E.,
Rojano-Delgado, A.M., Alves, P.L.C.A., De Prado, R. 2021. First
Case of Glyphosate Resistance in Bromus catharticus Vahl.: Examination
of Endowing Resistance Mechanisms. Frontiers in Plant Science 12:617945.
https://doi.org/10.3389/fpls.2021.617945
Cenchrus longispinus (Hack.) Fernald
Anderson, R. 1997. Longspine Sandbur (Cenchrus
longispinus) Ecology and Interference in Irrigated Corn (Zea mays). Weed
Technology 11(4), 667-671. https://doi.org/10.1017/S0890037X00043220
Naqinezhad, A.R. 2012. Cenchrus
longispinus (Poaceae), a new record from coastal sands of Caspian Sea (N
Iran)', Rostaniha 13(2), pp. 211-214. https://doi.org/10.22092/botany.2013.101338
Soltani, N., Kumagai, M., Brown, L.,
Sikkema, P.H. 2009. Long-spine sandbur [Cenchrus longispinus (Hack. in
Kneuck.) Fernald] control in corn. Canadian Journal of Plant Sciences
90, 241–45. https://doi.org/10.4141/CJPS09132
Strat, D., Stoyanov, S., Holobiuc, I.
2017. The occurrence of the alien plant species Cenchrus longispinus on
the Danube delta shore (Northwest black sea coast), threats and possible
impacts on the local biodiversity. – Acta Horti Botanici Bucurestiensis 44:
17-31.
Szigetvári, C. 2002. Distribution and
phytosociological relations of two introduced plant species in an open
grassland in the Great Hungarian Plain. Acta Botanica Hungarica
44, 163–83. https://doi.org/10.1556/ABot.44.2002.1-2.12
Verloove, F., Sanchez Gullon, E. 2012. A
taxonomic revision of non-native Cenchrus S.str. (Paniceae, Poaceae) in
the Mediterranean area. Willdenowia 42, 67–75. https://doi.org/10.3372/wi.42.42107
Chloris virgata P. Durand
Llewellyn, R.S., Ronning, D., Ouzman, J.,
Walker, S., Mayfield, A., Clarke, M. 2016. Impact of Weeds on Australian
Grain Production: The Cost of Weeds to Australian Grain Growers and the
Adoption of Weed Management and Tillage Practices. 2016. Report for GRDC.
CSIRO; Kingston, ACT, Australia. Available at: https://grdc.com.au/resources-and-publications/all-publications/publications/2016/03/impactofweeds
Mahajan, G., Chauhan, B.S. 2021. Evaluation
of Preemergent Herbicides for Chloris virgata Control in Mungbean. Plants
10, 1632. https://doi.org/10.3390/plants10081632
Manalil, S., Mobli, A., Chauhan, B.S.
2020. Competitiveness of windmill grass (Chloris truncata) and feathertop
Rhodes grass (Chloris virgata) in mungbean (Vigna radiata). Crop and Pasture
Science 71, 916–923. https://doi.org/10.1071/CP20092
Rachaputi, R.C.N., Sands, D., McKenzie,
K., Agius, P., Lehane, J., Seyoum, S. 2019. Eco-physiological drivers
influencing mungbean Vigna radiata (L.) Wilczek productivity in subtropical
Australia. Field Crops Research 238, 74–81. https://doi.org/10.1016/j.fcr.2019.04.023
Termeh, F. 2000. New Records of the
family Graminaeae from Iran. Rostaniha 1(1), pp. 43-62.
Cortaderia jubata
(Lemoine) Stapf
Drewitz, J.J., DiTomaso, J.M. 2004. Seed
biology of jubatagrass (Cortaderia jubata). Weed Science 52,
525–530. ttps://doi.org/10.1614/WS-03-081R
EPPO. 2019. Cortaderia jubata
(Lemoine ex Carrière) Stapf. EPPO Bulletin 49: 67–72. https://doi.org/10.1111/epp.12549
Lambrinos, J. G. 2000. The Impact of the
Invasive Alien Grass Cortaderia Jubata (Lemoine) Stapf on an Endangered
Mediterranean-Type Shrubland in California. Diversity and Distributions
6 (5): 217–31. http://www.jstor.org/stable/2673380
Stanton, A.E., DiTomaso, J.M. 2004.
Growth response of Cortaderia selloana and Cortaderia jubata
(Poaceae) seedlings to temperature, light and water. Madroño 51, 312–321.
Digitaria
longiflora (Retz.) Pers.
Galinato, M. I., Moody, K., Piggin, C. M. 1999. Upland Rice Weeds of South and Southeast Asia. International Rice Research Institute, Makati City (Philippines).
156 p.
Lapierre, H., Signoret, P.A. 2004. Viruses
and Virus Diseases of Poaceae (Gramineae). Editions INRA, Paris, France.
857 p. Available at: https://www.quae.com/extract/2218
Dinebra retroflexa
(Vahl) Panz.
Lapierre, H., Signoret, P.A. 2004. Viruses
and Virus Diseases of Poaceae (Gramineae). Editions
INRA, Paris, France. 857 p. Available at: https://www.quae.com/extract/2218
Mozaffarian, V. 1994. Studies on the
flora of Iran, new specie and new records. The Iranian Journal of Botany
6(2), pp. 235-244. https://doi.org/10.22092/ijb.2015.103309
Tanji A. 2020. Notes about two summer
annual grass weeds in Morocco: Dinebra retroflexa and Cenchrus
longispinus (Poaceae). Flora Mediterranea 30: 113-119. https://doi.org/10.7320/FlMedit30.113
Diplachne fusca (L.) P.Beauv. ex Roem. &
Schult.
Ghanbarpour, N., Zand, E., Sajedi, N.
2015. Efficacy of post-emergence herbicide for managing Diplachne fusca
in sugarcane field. Canadian Journal of Basic and Applied Sciences
03(04), 108-117.
Shu-zhong, Y., Yong-rui, C., Zhi-ming,
X., Jing-xuan, C., Yue-yang, C., Wei D. 2022. Influences of Diplachne fusca
(L.)Beauv. on growth and yield traits of rice and its eco-economic
threshold[J]. Hubei. Agricultural Science 61(6): 69-75.
McIntyre, S., Mitchell, D.S., Ladiges,
P.Y. 1989. Germination and seedling emergence in Diplachne fusca: a
semi-aquatic weed of rice fields. La Trobe. Journal contribution. https://doi.org/10.26181/22275232.v1
Ebrahmi, A., Ovisi M., Zand, E. 2017. Influence
of environmental factors on seed germination and seedling emergence of Leptochloa
fusca. Iranian Weed Science congress 2017.
Süveges, K., Molnár, A.V., Mesterházy,
A., Budai, J.T., Réka, F. 2021. Emergence of a new salt-tolerant alien grass
along roadsides? Occurrence of Diplachne fusca subsp. fascicularis
(Poaceae) in Hungary. Acta Botanica Croatica 80 (2), 140-145. https://doi.org/10.37427/botcro-2021-014
Saito, K. 2010. Weed pressure level and
the correlation between weed competitiveness and rice yield without weed
competition: An analysis of empirical data. Field Crop Research 117,
1–8. https://doi.org/10.1016/j.fcr.2010.02.009
Echinochloa
oryzoides (Ard.)
Fritsch
Avarseji, Z. 2015. Characterizing the
competitive traits of watergrass (Echinochloa oryzoides) as a
new-introduced, and barnyardgrass (E. crus-galli) as a common weed
species in rice. Journal of Plant Production Research 22(3), pp.
224-241.
Fischer, A., Ateh, C., Bayer, D., Hill,
J. 2000. Herbicide-resistant Echinochloa oryzoides and E. phyllopogon
in California Oryza sativa fields. Weed Science 48(2), 225-230. https://doi.org/10.1614/0043-1745(2000)048[0225:HREOAE]2.0.CO;2
Haghnama, K., Mennan, H. 2020. Herbicide
resistant barnyardgrass in Iran and Turkey. Planta Daninha [Internet].;38:e020227592.
Available from: https://doi.org/10.1590/S0100-83582020380100060
Hill, J. E., Carriere, M.D., Cook, J.F.,
Butler, T.D., Lana, P.J., Hare, J. 1994. Londax resistance management
strategies for California rice. In: Proceedings of the California Weed
Conference, v. 46., pp. 180–185 Freemont, CA, USA.
Pouramir, F., Yaghoubi, B. 2021. Biology
and management of the invasive (Echinochloa oryzoides (Ard.) Fritsch)
and common (Echinochloa crus-galli (L.) Beauv.) barnyardgrass in paddy
field. Iranian Journal of Weed Science 17(1), pp. 71-84. https://doi.org/10.22092/ijws.2020.128351.1357
Zand, E., Baghestani, M.A., Nezamabadi, N., Shimi, P, Mousavi, S.K.
2019. A guide for herbicides in Iran. University
Press Center, 216pp. [In Persian]
Eragrostis curvula (Schrad.) Nees
Brown, J., Merchant, A. & Ingram, L.
2023. Utilising random forests in the modelling of Eragrostis curvula
presence and absence in an Australian grassland system. Scientific Reports
13, 16603. https://doi.org/10.1038/s41598-023-43667-w
Firn, J. 2009. African lovegrass in
Australia: a valuable pasture species or embarrassing invader? Trop Grassl
43:86–97
Firn, J., Price, J.N., Whalley, R. D. 2013. Using strategically applied
grazing to manage invasive alien plants in novel grasslands. Ecological
Processes 2: 26. https://doi.org/10.1186/2192-1709-2-26
Firn, J., Ladouceur, E., Dorrough, J.
2017. Integrating local knowledge and research to refine the management of an
invasive non-native grass in critically endangered grassy woodlands. Journal
of Applied Ecology 55. https://doi.org/10.1111/1365-2664.12928
Godfree, R., Firn, J., Johnson, S.,
Knerr, N., Stol, J., Doerr, V. 2017. Why non-native grasses pose a critical
emerging threat to biodiversity conservation, habitat connectivity and
agricultural production in multifunctional rural landscapes. Landscape
Ecology 32. https://doi.org/10.1007/s10980-017-0516-9
Johnston, W.H., Cornish, P.S., Shoemark,
V.F. 2005. Eragrostis curvula (Schrad.) Nees. complex pastures in
southern New South Wales, Australia: a comparison with Medicago sativa
L. and Phalaris aquatica L. pastures under rotational grazing. Animal
Production Science 45:401–420. https://doi.org/10.1071/EA03117
Leersia oryzoides (L.) Sw.
Deaver, E., Moore, M.T., Cooper, C.M.,
Knight, S.S. 2005. Efficiency of three aquatic macrophytes in mitigating
nutrient runoff. International Journal of Ecology and Environmental Sciences
31: 1–7.
Koontz, M.B., Pezeshki, S.R. 2011. Rice
cutgrass growth as affected by simulated flooding and water nitrogen
concentration under greenhouse conditions. Journal of Soil and Water
Conservation 66: 329–336. https://doi.org/10.2489/jswc.66.5.329
Mozaffarian, V. 1991. New Species and new
plant records from Iran. The Iranian Journal of Botany 5(1), pp. 29-39.
Pierce, S., Pezeshki, S.R., Moore, M.T.
2007. Ditch plant response to variable flooding: A case study of Leersia
oryzoides (rice cutgrass). Journal of Soil and Water Conservation
62: 216–225. https://doi.org/10.1080/00224561.2007.12435955
Pierce, S.C., Pezeshki, S.R., Larsen, L.,
Moore, M.T. 2009. Hydrology and species-specific effects of Bacopa monnieri
and Leersia oryzoides on soil and water chemistry. Ecohydrology 2:
279–286. https://doi.org/10.1002/eco.54
Leptochloa chinensis (L.) Nees
Dong, F., Xu, J., Zhang, X., et al. 2020.
Gramineous weeds near paddy fields are alternative hosts for the Fusarium
graminearum species complex that causes fusarium head blight in rice. Plant
Pathology 69: 433–441. https://doi.org/10.1111/ppa.13143
Hayyat, M., Safdar, M., Javaid, M.,
Ullah, S., Chauhan, B. 2023. Estimation of the economic threshold of Leptochloa
chinensis (Chinese sprangletop) in direct-seeded fine grain rice (Oryza
sativa). Semina: Ciencias Agrarias 44. 803-822. https://doi.org/10.5433/1679-0359.2023v44n2p803
IRRI. 2023. Leptochloa chinensis. http://www.knowledgebank.irri.org/training/fact-sheets/item/leptochloa-chinensis
Pane, H., Mansor, M., Watanabe, H. 1996.
Yield component analysis of direct seeded rice [Oryza sativa] under
several densities of red sprangletop (Leptochloa chinensis (L.) Nees) in
peninsular Malaysia. Weed Research (Japan). https://doi.org/10.3719/weed.41.216
Peng, Y., Pan, L., Liu, D., Cheng, X.,
Ma, G., Li, S., Liu, X., et al. 2020. Confirmation and
characterization of cyhalofop-butyl–resistant Chinese sprangletop (Leptochloa
chinensis) populations from China. Weed Science 68(3), 253-259. https://doi.org/10.1017/wsc.2020.15
Microstegium vimineum (Trin.) A. Camus
Bor, N.L.1970. Graminae in Rechinger, K.
H. (ed.), Flora Iranica. no. 70— Graz Akademische Druckund
Verlagsanstalt.
Hamzeh'ee, B., Naqinezhad A. 2009. Arthraxon P. Beauv (Gramineae) and Carex
caryophyllea (Cyperceae): New genus and species records from Iran. Iranian
Journal of Botany. 15 (1): 68-71. Tehran.
Johnson, D.J., Flory, S.L., Shelton, A.,
Huebner, C., Clay, K. 2015. Interactive effects of a non-native invasive grass Microstegium
vimineum and herbivore exclusion on experimental tree regeneration under
differing forest management. Journal of Applied Ecology 52: 210-219. https://doi.org/10.1111/1365-2664.12356
Zhao, W., Brenner, E.D. 2021. Nodding
behavior observed in Japanese stiltgrass, Microstegium vimineum,
seedlings from time-lapse observations. Plant Signaling & Behavior 16(12):2010317.
https://doi.org/10.1080/15592324.2021.2010317; PMID: 35139001; PMCID: PMC9208775.
Oryza
sativa f. spontanea Roshev.
Cao, Q.J., Li, B.O., Zhi, P.S., Xing,
X.C., Bao Rong, L. 2007. Impact of weedy rice populations on the growth and
yield of direct seeded and transplanted rice. Weed Biology and Management
7(2): 97-104. https://doi.org/10.1111/j.1445-6664.2007.00241.x
Galvin, L.B., Inci, D., Mesgaran, M.,
Brim-DeForest, W., Al-Khatib, K. 2022. Flooding depths and burial effects on
seedling emergence of five California weedy rice (Oryza sativa spontanea)
accessions. Weed Science 70(2):213-219 https://doi.org/10.1017/wsc.2021.82
Takasi, S, Fakhari, R., Sharifi Ziveh, P.
2021. Introduction of problematic rice weed in direct cultivation of rice. Rice
Extension Journal 3(2), 1-5.
Oryza
rufipogon Griff.
Amarasinghe, Y., Otsuka, M., Lim, S.,
Ishikawa, R., Ishii, T. 2020. The role of wild rice (Oryza rufipogon)
awns in seed dispersal. Seed Science Research 30(4), 319-324. https://doi.org/10.1017/S096025852000046X
Gupta, S.R., Upadhyay, M.P. 2000. Wild
and weedy rice in the Nepalese ecosystem. Rungsit Suwanketnikon 41:
55-58.
Jamjod, S., Maneechote, C.,
Nirantrayakul, S. and Rerkasem, B. 2005. The good and bad gene flow in the rice
landscape. In: The International Symposium on Diversity, Management,
Protection and Utilization of Local Rice Germplasm. Chiang Mai, Thailand.
August 1-5, 2005, 97-105.
Na Chiangmai, P., Yodmingkhwan, P. 2011.
Competition of root and shoot growth between cultivated rice (Oryza sativa
L.) and common wild rice (Oryza rufipogon Griff.) grown under different
phosphorus levels. Songklanakarin Journal of Science and Technology 33.
685-692.
Panicum
capillare L.
Clements, D.R., DiTommaso, A.,
Darbyshire, S.J., Cavers, P.B., Sartonov, A.D. 2004. The biology of Canadian
weeds. 127. Panicum capillare L. Canadian Journal of Plant Science
84, 327–341. https://doi.org/10.4141/P02-147
Kingsbury, J. M. 1964. Poisonous
Plants of the United States and Canada. Prentice Hall. Englewood Clifs, NJ,
USA. https://doi.org/10.1097/00010694-196411000-00022
Ontario Weed Committee. 2021. Witch
Grass, Panicum capillare. Available online at: http://www.weedinfo.ca/en/weed-index/view/id/PANCA
[accessed on April 30, 2023].
Quinn, J.C., Kessell, A.E., and Weston,
L.A. 2014. Secondary plant products causing photo-sensitization in grazing
herbivores: their structure, activity, and regulation. International Journal
of Molecular Sciences 15, 1441–1465. https://doi.org/10.3390/ijms15011441
Termeh, F. 2000. New records of the
family gramineae from Iran (3), Rostaniha 1(1), pp. 43-62.
Panicum repens L.
Akamine, H., Hossain, M.A., Ishimine, Y.,
Kuramoch, H. 2007. Bud sprouting of torpedograss (Panicum repens L.) as
influenced by the rhizome moisture content. Weed Biology and Management
7: 188-191. https://doi.org/10.1111/j.1445-6664.2007.00255.x
Chandrasena, J.P.N.R., Peiris, H.C.P.
1989. Studies on the biology of Panicum repens L. II. Intraspecific
competition and resource‐allocation. Tropical Pest Management 35:3, 316-320, https://doi.org/10.1080/09670878909371389
Hossain, M.A. 1996. Competition
between sugarcane (Saccharum officinarum L.) and torpedograss (Panicum repens
L.). M.S. dissertation. University of the Ryukyus, Japan. 207 p.
Hossain, M.A., Ishimine, Y., Akamine, H.,
Murayama, S., Uddin, S.M.M., Kuniyoshi, K. 1999. Effect of Burial Depth on
Emergence of Panicum Repens. Weed Science 47: 651–56. https://doi.org/10.1017/S0043174500091281
Stephenson, D.O., Brecke, B.J., Unruh,
J.B. 2006. Control of Torpedograss (Panicum repens) with
Trifloxysulfuron-Sodium in Bermudagrass (Cynodon dactylon × Cynodon
transvaalensis) Turf. Weed Technology 20(2), 351-355. https://doi.org/10.1614/WT-05-051R2.1
Paspalum
dilatatum Poir.
Ala, A., AghaAlikhani, M., Amiri
Larijani, B., Soufizadeh, S. 2014. Comparison Between
Direct-seeding and Transplanting of Rice in Mazandaran Province: Weed
Competition, Yield and Yield Components. Iranian Journal of Field Crops
Research 12(3), pp. 463-475. https://doi.org/10.22067/gsc.v12i3.22637
Bondy, G.S., Voss, K.A., Haschek, W.M.
2023. Mycotoxins. In: Haschek, W.M., Rousseaux, C.G., Wallig, M.A., Bolon, B.,
Heinz-taheny, K.M., Rudmann, D.G., Mahler, B.W. (Eds.), Haschek and
Rousseaux' s Handbook of Toxicologic Pathology, Chapter 6, pp. 393-488 -
(Fourth Edition), Academic Press. https://doi.org/10.1016/B978-0-443-16153-7.00006-X
Faghih, Z., keshavarzi, M., Mahmoodi
Otaghvari, A., Mosaferi, S. 2020. Systematic study of Paspalum
(Poaceae) species in Iran. Iranian Journal of Plant Biology 12(3), pp.
43-56. https://doi.org/10.22108/ijpb.2020.121656.1201
Golmohammadi, M.J., Mohamadduost, H.,
Yaghoubi, B., Oveisi, M. 2019. Study of Indices of Weed
Communities in Rice Fields of Guilan Province. Journal of Iranian Plant
Protection Research 33(1), pp. 69-84. https://doi.org/10.22067/jpp.v33i1.67543
Jacobo, E., Rodríguez, A., Durand, M.,
Deregibus, V. 2009. Sowing date and nitrogen supply
determine the outcome of competition between dallisgrass (Paspalum dilatatum
Poir.) and tall fescue (Festuca arundinacea Schreb.) in the Pampas
region of Argentina. Grass and Forage Science 64. 71 - 79. https://doi.org/10.1111/j.1365-2494.2008.00669.x
Napier, J.D., Mordecai, E.A., Heckman,
R.W. 2016. The role of drought- and disturbance-mediated competition in shaping
community responses to varied environments. Oecologia 181(2):621-32. https://doi.org/10.1007/s00442-016-3582-9
Epub 2016 Feb 18. PMID: 26893230.
Nasseri, A. 2016. Canal geometry, flow
velocity, dallisgrass (Paspalum dilatatum Poir.) density and soil
phosphorous effects on hydraulic resistance of vegetated canals. Tarim Bilimleri Dergisi 22(2) 187-195. http://tarimbilimleri.agri.ankara.edu.tr/2016/22_2/6.makale%20(1).pdf
Tejera, M., Speranza, P., Astigarraga, L., Picasso, V.
2016. Forage biomass, soil cover, stability and competition in
perennial grass–legume pastures with different Paspalum species. Grass and
Forage Science 71: 575-583. https://doi.org/10.1111/gfs.12208
Paspalum
distichum L.
Bernez, I., Ferreira, M.T., Albuquerque, A., Aguiar, F.
2005. Relations between river plant richness in the Portuguese
floodplains and the widespread water knotgrass (Paspalum paspalodes).
Hydrobiologia, French Limnological Association conference 'Biodiversity of
aquatic ecosystems', Paul Verlaine - Metz University, France, December 2003.,
551:121-130. https://doi.org/10.1007/s10750-005-4454-1
Faghih, Z., keshavarzi, M., Mahmoodi
Otaghvari, A., Mosaferi, S. 2020. Systematic study of Paspalum (Poaceae)
species in Iran. Iranian Journal of Plant Biology 12(3), pp. 43-56. https://doi.org/10.22108/ijpb.2020.121656.1201
Kojima, K., Kawana, Y., Ushiki, J. 2005. Growth of two perennial grass weeds (Paspalum distichum L. and
Leersia japonica Makino) under different weed control levels in rice. Journal
of Weed Science and Technology 50. 104-105. https://doi.org/10.3719/weed.50.Supplement_104
Kumar, C.R.A., Mittal, D.D. 1993. Habitat
preference of fishes in wetlands in relation to aquatic vegetation and water
chemistry. Journal of the Bombay Natural History Society 90(2):181-192.
Ladmakhi-nezhad, A.H., Mohammadvand, E.,
Asghari, J. 2023. Influence of duration of inter-species interference and
determination of the critical period for weed control in peppermint (Mentha
piperita L.). Journal of Iranian Plant Protection Research 37(3), pp. 301-314. https://doi.org/10.22067/jpp.2023.71514.1035
Le, P., Denich, M., Vlek, P., Balasubramanian, V. 2005. Suppressing Weeds in Direct‐seeded Lowland Rice: Effects of
Methods and Rates of Seeding. Journal of Agronomy and Crop Science 191,185 -
194. https://doi.org/10.1111/j.1439-037X.2005.00151.x
Phuong, L.T., Denich, M., Vlek, P.L.G., Balasubramanian,
V. 2005. Suppressing Weeds in Direct-seeded Lowland Rice: Effects of Methods
and Rates of Seeding. Journal of Agronomy and Crop Science 191: 185-194.
https://doi.org/10.1111/j.1439-037X.2005.00151.x
Stroh, H.G. 2006. Contribution to the
ephemeral wetland vegetation along riverbanks and lakeshores of Western Thrace
(NE Greece). (Beitrag zur Therophytenvegetation an Fluss- und Seeufern in
West-Thrakien (NO-Griechenland).) Tuexenia No.26:353-388. http://www.tuexenia.de
Paspalum
urvillei Steud.
Ghorbani, Z., Ghahremaninejad, F.,
Tavakkoli, Z. 2021. Flora of Kharazmi University Campus, Karaj, Alborz
province, Iran. Journal of Plant Research (Iranian Journal of Biology)
34(1), pp. 144-156.
Jeffries, M.D., Gannon, T.W., Yelverton,
F.H. 2017. Herbicide inputs and mowing affect vaseygrass (Paspalum urvillei)
control. Weed Technology 31, 120–129. https://doi.org/10.1614/WT-D-16-00072.1
Rottboellia cochinchinensis (Lour.) Clayton
Bolfrey-Arku, G.E.-K., Chauhan, B.S.,
Johnson, D.E. 2011. Seed germination ecology of itchgrass (Rottboellia
cochinchinensis). Weed Science 59, 182-187. https://doi.org/10.1614/WS-D-10-00095.1
Correia, N.M. 2016. Biology and
management of Rottboellia cochinchinensis. Revista Brasileira
de Herbicidas 15(1):89-96. https://doi.org/10.7824/rbh.v15i1.437
Saccharum
spontaneum L.
Bonnett, G., Kushner, J., Saltonstall, K.
2014. The reproductive biology of Saccharum spontaneum L.: implications
for management of this invasive weed in Panama. NeoBiota 20: 61-79. https://doi.org/10.3897/neobiota.20.6163
Holm, L., Doll, J., Holm, E., Pancho, J.,
Herberger, J. 1997. World Weeds. Natural Histories and Distribution.
John Wiley and Sons, Inc., New York, USA.
Waterhouse, D.F. 1994. Biological Control
of Weeds: Southeast Asian Prospects. Canberra, Australia: ACIAR
Monograph No 26.
Setaria italica
(L.) P.Beauv.
Abbaspoor, M. 2020. Chemical Weed Control
in Foxtail Millet (Setaria italica L.). Journal of Plant Production
Research 26(4): 149-162. https://doi.org/10.22069/jopp.2019.15731.2410
Darmency, H., Zangre, G.R., Pernes, J. 1987.
The wild-weed-crop complex in Setaria: a hybridization study. Genetica
75, 103–107. https://doi.org/10.1007/BF00055253
Dawson, J.H., Dell’Agostino, E. 1978. Control
of Foxtail Millet (Setaria Italica) in New Seedings of Alfalfa (Medicago
Sativa) with EPTC Applied in Surface Lines. Weed Science 26(6), 637–39. https://doi.org/10.1017/S0043174500064717
Dekker, J. 2003. The foxtail (Setaria)
species-group. Weed Science 51(5), 641-656. https://doi.org/10.1614/P2002-IR
Setaria
parviflora (Poir.) Kerguélen
Austin, D.F. 2006. Foxtail millets (Setaria:
Poaceae)—Abandoned food in two hemispheres. Econ Bot 60, 143–158. https://doi.org/10.1663/0013-0001(2006)60[143:FMSPFI]2.0.CO;2
Doust, A.N., Kellogg, E.A. 2002. Infl
orescence diversifi cation in the panicoid “bristle grass” clade (Paniceae,
Poaceae): Evidence from molecular phylogenies and developmental morphology. American
Journal of Botany 89: 1203 – 1222. https://doi.org/10.3732/ajb.89.8.1203
Dyer, L., Henry, G., McCullough, P.,
Belcher, J., Basinger, N. 2022. Knotroot Foxtail [Setaria parviflora
(Poir.) Kerguélen]: “A sly fox”. Weed Technology 36(6), 891-897. https://doi.org/10.1017/wet.2022.101
Meksawat, S., Pornprom, T. 2010. Allelopathic effect of itchgrass (Rottboellia cochinchinensis)
on seed germination and plant growth. Weed Biology and Management 10:
16-24. https://doi.org/10.1111/j.1445-6664.2010.00362.x
Millholon, R.W. 1992. Effect of Itchgrass
(Rottboellia Cochinchinensis) Interference on Growth and Yield of
Sugarcane (Saccharum Spp. Hybrids). Weed Science 40(1), 48–53. https://doi.org/10.1017/S0043174500056939
![]() , Javid
Gherekhloo2
, Javid
Gherekhloo2 ![]() , Jose L. Gonzalez-Andujar3
, Jose L. Gonzalez-Andujar3 ![]() ,
Montserrat Vilà4,5
,
Montserrat Vilà4,5 ![]()

