Introduction
Scleria
P.J.Bergius (1765: 142), commonly known as nut rushes or razor grasses, is the
sixth largest genus in the Cyperaceae family with around 260 species (Bauters et al.
2016; Larridon et al. 2021;
2024), and the only genus of tribe Sclerieae Wight & Arn. (subfamily
Cyperoideae) (Larridon 2022). Scleria has four subgenera and 17
sections (Bauters
et al. 2016, 2018a, 2019). Most
species occur in tropical areas but some occupy more temperate climatic regions
(Larridon et
al. 2021). Scleria
is a functionally diverse genus that includes annual and perennial herbs, the
latter often using stoloniferous rhizomes or tubers (Bauters
et al. 2016; Galán Díaz et al. 2019). Nut
rushes are ecologically important in wetlands and areas undergoing secondary
succession (Bauters et al. 2016), and some
species are reported to have local economic significance (Simpson y Inglis 2001; Galán Díaz et
al. 2024).Recent studies have disentangled the
evolutionary relationships among Scleria species (Bauters et al. 2016, 2018) and identified major dispersal and niche
shift events (Larridon et
al. 2021). Still, we lack a
global assessment of species and world regions of remarkable interest for Scleria
conservation.
In the current context of rapid global
change, there is a need to adopt new tools to assess biodiversity and
ecosystems to set conservation priorities (Cowell
et al. 2022). Over the
last two decades, the use of phylogenetic and functional indices has emerged as
a powerful approach to support conservation assessments by identifying at-risk
species and regions of great evolutionary and ecological uniqueness (Kondratyeva
et al. 2019). The
Evolutionarily Distinct and Globally Endangered (EDGE) metric can be used to
rank species conservation priorities where species’ evolutionary
distinctiveness (ED) is used as a surrogate of their irreplaceability, and
species’ probability of extinction (GE) is used as a proxy of their
vulnerability (Isaac et al.
2007). It has been
successfully implemented in different groups such as corals (Huang 2012), mammals and amphibians (Safi et al.
2013), gymnosperms (Forest
et al. 2018) and
tetrapods (Gumbs et al.
2018). The EDGE metric has
been recently revised to include new advances (EDGE2; Gumbs et
al. 2023): (i) species’
evolutionary distinctiveness (ED2) now considers the extinction risk of their
close relatives; (ii) it allows the incorporation of uncertainty in the
phylogeny and extinction risk. The latter allows the use of phylogenies which
have been expanded using non-molecular information (e.g., see Ramos-Gutiérrez et al. 2023), and provides a flexible framework to assess Not Evaluated and
preliminary assessed species rather than only depending on species published in
the Red List of Threatened Species of the International Union for Conservation
of Nature (IUCN) (e.g., Walker et
al. 2023).
Machine learning approaches constitute
efficient tools to produce extinction risk assessments and their implementation
in different plant groups has provided promising results (Zizka et al.
2022; Bachman et al. 2023; Walker et al. 2023). The latest version of the Red List
includes ~18% (62 666 species) of all known vascular plant species (IUCN 2023). In the case of Scleria, there are
extinction risk assessments available for 183 species (26 of which are not yet
publicly available in the latest version of the Red List), which accounts for
70% of the genus. Recent taxonomic revisions of the genus (Bauters
et al. 2016, 2018a, 2019; Galán Díaz et al. 2019) and the availability of occurrence
datasets curated using expert knowledge (Larridon et
al. 2021) can help overcome
some of the limitations and challenges that arise from using herbarium
collections to support preliminary extinction risk assessments (Nic Lughadha
et al. 2019). The
genus Scleria is therefore a good study system to train machine learning
algorithms and produce preliminary extinction risk assessments.
Finally, the phylogenetic and functional
components of biodiversity are not necessarily correlated, especially when
considering functional attributes which are highly dependent on the environment
(Losos 2008). Scleria is an ecologically diverse
clade that includes species with variable habits, from tiny annuals with small
nutlets and fibrous roots to climbers or stout perennials with big buoyant
propagules (Galán Díaz
et al. 2019). The
variation in functional traits across the genus Scleria therefore
warrants independent evaluation. In this regard, the EDGE2 framework can
incorporate species functional distinctiveness (FUD) as a measure of ecological
irreplaceability. This metric is termed Ecologically Distinct and Globally
Endangered (EcoDGE) (Hidasi-Neto et al. 2015).
Here, (i) we first produced preliminary
extinction risk assessments for the ~30% of Scleria species that do not
yet have a global Red List assessment, and then (ii) followed the EDGE2
protocol to identify evolutionary and ecologically distinct Scleria
species at greatest risk of extinction and conservation priority areas. For
this, we used extinction risk assessments from the global Red List and the most
comprehensive phylogeny of Scleria to date (Larridon et
al. 2021), as well as newly
compiled occurrence and traits datasets.
Material and Methods
Taxonomy and species occurrences
We used the World Checklist of Vascular
Plants (WCVP; Govaerts
et al. 2021) and the latest
taxonomic revisions of the genus Scleria P.J.Bergius (Bauters
et al. 2016, 2019) to compile
a dataset of 261 accepted species. Data relating to infraspecific taxa were
incorporated at the species level. Species names and authorities are available
in in Table A1 of Appendix.
Occurrence data was compiled using
observations from the Global Biodiversity Information Facility (GBIF 2023), Red List (IUCN 2023), research-grade identifications from iNaturalist and
records for collections from BR, K, GENT, L, MO, NY, P, US and WAG which were
georeferenced using Google Earth. For each species, we filtered observations
following several steps: (1) we removed duplicates and retained one observation
per 1km2 raster cell, (2) filtered observations based on the native
ranges at the ‘botanical country’ scale, according to the WCVP, and (3)
excluded observations 1.5 times outside the interquartile climatic range using
the mean annual temperature and annual precipitation BIOCLIM variables (Booth et al.
2014). The final occurrence
database included 22 759 observations from 248 species. We projected locality
data using the Equal Earth map projection (Šavrič
et al. 2019).
Traits measurements and
calculation
We measured maximum height, maximum blade
length, maximum blade width, nutlet length and nutlet width from 1254 specimens
housed at Royal Botanic Gardens, Kew (K) and the Muséum National d'Histoire
Naturelle in Paris (P). For species missing in these herbaria, we completed the
dataset using information from protologues and descriptions from regional
floras (e.g., Davidse et al. 1994; Simpson and
Koyama 1998; Le Roux 2015).
This information was used to estimate three traits informative of plant
ecological strategies (Westoby 1998): maximum height (i.e., distance from the
top inflorescence to the ground), leaf area and size of the propagule (i.e.,
nutlet volume). We estimated leaf area and nutlet volume using the formula of
an ellipse and an ellipsoid, respectively. We also considered plant longevity
(annual/perennial).
Extinction risk data and
preliminary assessments
Previous studies have shown that the Red
List is biased toward certain plant groups and species, for instance, woody
perennials and useful plants (Nic Lughadha et al. 2020). Still, we
used the Red List categories as indicators of species’ extinction risk because
(i) the Red List constitutes a consensual framework worldwide, (ii) the authors
have been continuously involved in the production of several Red List
assessments of Scleria over the last decade which facilitates the
interpretation of the results, (ii) Scleria is nearly comprehensively
assessed according to the Red List criterium (i.e., taxa with over 149 species
with at least 80% of the species assessed) and (iv) recent advances in the EDGE
protocol used Red List categories as proxies of species’ probability of
extinction (Gumbs et al. 2023)
We downloaded 157 extinction risk
assessments from the Red List (version 2023-1) and considered the extinction
risk category of other 26 species that have assessments in preparation. To
supplement the 183 species with global (published and in preparation) Red List
assessments we carried out preliminary assessments and predictions for the 67
Data Deficient (DD) and Not Evaluated (NE) species for which occurrence data
was available so that we had an extinction risk assessment for all species. The
extinct species S. chevalieri J.Raynal was excluded from the analyses.
We followed two approaches:
·
We estimated species extinction risk categories
under IUCN Red List criterion B using the function ‘ConBatch’ from the R
package ‘rCAT’ (Moat & Bachman
2020). Given a dataset of occurrences, this function provides preliminary
extinction risk categories based on species’ extent of occurrence (EOO). We did
not consider the species' area of occupancy (AOO) because it can lead to an
overestimation of extinction risk when using occurrence data derived from
herbarium records (Nic Lughadha
et al. 2019). To
assess the accuracy of this method, we ran ‘ConBatch’ on the species included
in the Red List and compared the actual assessments with the predicted
categories.
·
We implemented a machine learning algorithm
(random forest, henceforth RF) using as predictors: EOO, AOO, mean of
latitudinal range, elevation, minimum human population density in 2020 (HPD; CIESIN 2016),
human footprint index in 2013 (HFI; Mu et a. 2022), proportion of
observations located in protected areas (UNEP-WCMC y
IUCN 2023), mean annual
temperature, minimum temperature of the coldest month, temperature annual
range, annual precipitation, precipitation of the driest month and
precipitation seasonality. All predictors were resampled at 30 seconds. For HPD
and HFI, we calculated the average index value within a 5 km circular buffer of
each unique occurrence point. We used 10 repeats of 5-fold cross-validation to
train and evaluate the model and retained 20% of data for external validation.
EDGE2 and EcoDGE calculation and
species lists
We computed two metrics that combine
species extinction risk with their evolutionary or functional distinctiveness
to prioritize at-risk Scleria species and world regions of special
interest for conservation: the Evolutionarily Distinct and Globally Endangered
metric (EDGE2) (Gumbs et al.
2023) and the Ecologically
Distinct and Globally Endangered metric (EcoDGE) (Hidasi-Neto et al. 2015). To compute ED2, we used the latest
phylogenetic inference of the genus from Larridon et al. (2021b), which included
136 species. We used the R package ‘randtip’ (Ramos-Gutiérrez
et al. 2023) to impute 111 species missing in
the phylogeny but for which infrageneric information (i.e., section) was
available. We excluded 14 species for which infrageneric information was not
available. Trees were expanded following several criteria: species were imputed
in their sections at random, the probability of branch selection was set as
equiprobable, and the stem branch was considered as a candidate for binding.
This step was repeated to generate a distribution of 500 randomly imputed
phylogenetic trees.
To calculate FUD, we adapted the EcoDGE
framework proposed by Hidasi-Neto et al. (2015) to the new
EDGE2 protocol following Griffith
et al. (2022). We generated a dendrogram by
calculating pairwise species dissimilarities with a generalized Gower's
distance matrix (Gower 1971) using the four traits (i.e., longevity,
maximum height, leaf area and size of the propagule) and an unweighted pair
group method with arithmetic mean (UPGMA). We repeated the dendrogram
construction 11 times (all possible combinations of two, three and four traits)
to reduce the impact of specific traits on the results. Finally, we multiplied
FUD scores by 100 to balance the weighting of the two component values of the
EcoDGE metric.
The EDGE2 and EcoDGE protocols allow the
incorporation of uncertainty in the quantification of extinction probabilities
by associating each Red List category to a distribution of extinction
probabilities based on a 50-year time horizon (Mooers et al. 2008). For species not included in the Red List and classified as
threatened by the preliminary assessments, the extinction risk component
(GE) was randomly drawn from CR, VU and EN categories. For species classified
as non-threatened, GE values were randomly withdrawn from those of NT and LC
categories.
Finally, for each species, we calculated a
distribution of EDGE2 and EcoDGE scores (N=500) which were used to sort Scleria
species in three lists as proposed by Gumbs et al. (2023): a ‘priority or main
EDGE2 species list’ that includes threatened species whose distribution of
EDGE2 scores rank above the median of the entire genus at least 95% of the
times; a ‘borderline EDGE2 species list’ that includes threatened species whose
distribution of EDGE2 scores rank above the median of the entire genus at least
80% of the times; and a ‘watch EDGE2 species list’ that includes nonthreatened
species whose distribution of EDGE2 scores rank above the median of the entire
genus at least 95% of the times. We further discuss the results in the context
of the countries and world terrestrial ecoregions (Olson et al.
2001), which are good
descriptors of plant distribution (Smith et al.
2018) and can be used as
informative units for conservation planning (Dinerstein
et al. 2017).
All analyses were performed in R (version
4.3.2).
Results
Preliminary assessments
rCAT and RF yielded different results (Table A2 of Appendix). Under criterion B, rCAT classified 53 out of the 67 non-assessed
species as threatened according to their EOO (44 CR, 5 EN, 4 VU, 4 NT, 10 LC). Scleria
rCAT assessments correctly classified 79.31% of threatened species (high
sensitivity) and 91.39% of non-threatened species (high specificity). RF
classified 39 species as threatened and 28 as non-threatened. It achieved an
accuracy of 97.22%, correctly classifying 96.55% of non-threatened species and
all threatened species (higher sensitivity and specificity than rCAT). The
single most important predictor for RF was EOO, followed by AOO. Because of its
greater performance, we retained the results of RF for the EDGE2 and EcoDGE
calculations. Combined Red List results (published, unpublished and
preliminary) are available in Table
A1 of Appendix.
Considering
the published (Red List version 2023-1; IUCN 2023) and preliminary (RF) results,
we found that the Afrotropics and the Neotropics had the highest number and
proportion of threatened species (Fig. 1a). The sections with the
greatest proportion of threatened species were Hypoporum (41.54%),
followed by Abortivae, Browniae, Schizolepsis and Foveolidia (28.57%) (Fig. 1b).
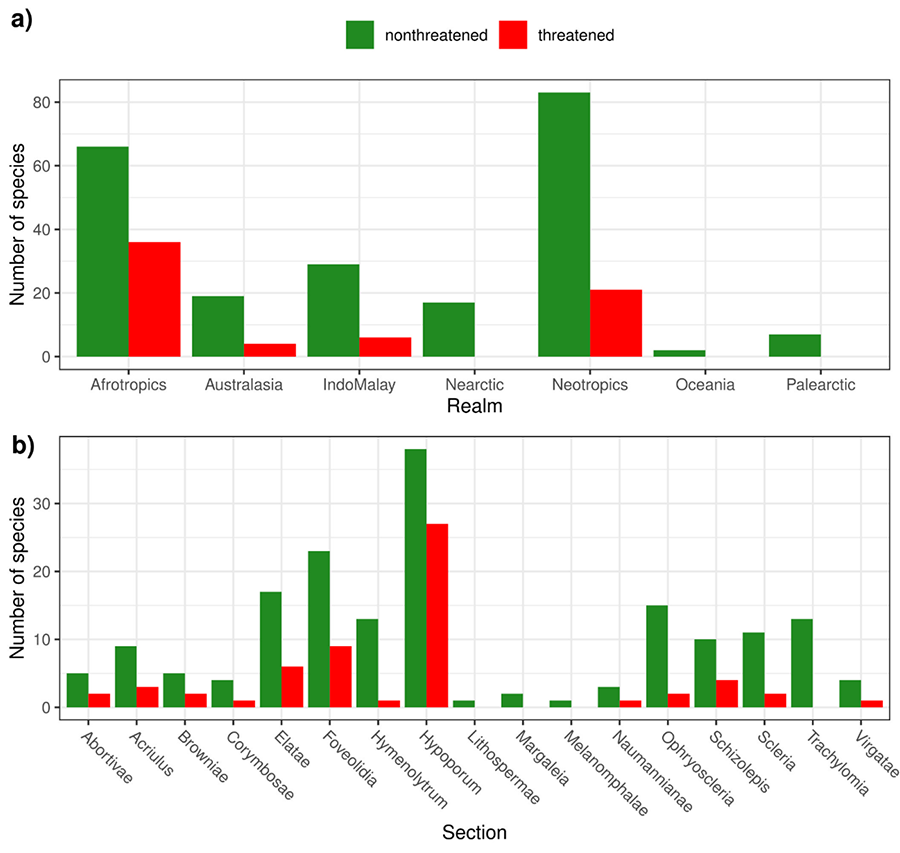
Figure 1. Number of threatened and non-threatened Scleria species
grouped by (a) Realm (Olson et al.
2001) and (b)
Section. Threatened refers to species classified as Vulnerable (VU), Endangered
(EN) and Critically Endangered (CR) in the Red List, as well as Data Deficient
(DD) and Not Evaluated (NE) species classified as threatened by the random
forest approach. Non-threatened refers to species classified as Least Concern
(LC) and Near Threatened (NT) in the Red List, as well as Data Deficient (DD)
and Not Evaluated (NE) species classified as non-threatened by the random
forest approach.
Figura 1. Número de especies de Scleria
amenazadas y no amenazadas agrupadas por (a) Reino biogeográfico (Olson et al. 2001) y (b) Sección. Amenazadas
agrupa especies clasificadas como Vulnerable (VU), En Peligro (EN) y En Peligro
Crítico (CR) en la Lista Roja, así como especies en las categorías No Evaluado
(NE) y Datos Insuficientes (DD) clasificadas como amenazadas por el random forest.
No amenazadas se refiere a las especies clasificadas como Preocupación Menor
(LC) y Casi Amenazado (NT) en la Lista Roja, así como a las especies en las
categorías No Evaluado (NE) y Datos Insuficientes (DD) clasificadas como no
amenazadas por el random forest.
Evolutionarily
and ecologically distinct species at greatest risk of extinction
The phylogenetic diversity (PD) across
trees was 900.96 ± 24.01 MY (mean ± SD). Species with the highest mean ED2
scores were Scleria corymbosa,
Scleria lithosperma and Scleria melanomphala with 22.28, 20.89
and 19.04 MY, respectively. The species with the
greatest mean EDGE2 score was Scleria
porphyrocarpa with
3.97 MY of avertable expected PD loss, followed by Scleria zambesica (2.53 MY), Scleria pulchella (2.25 MY) and Scleria madagascariensis (2.04 MY).
We detected 45
EDGE2 Scleria species (17.24% of species in the genus). Safeguarding
these species, we would secure 68.24% (41.49 MY) of avertable expected PD loss
in a 50-year time horizon. The main list included 23 species (Table 1), of
which 17 belong to section Hypoporum, three to section Acriulus,
two to section Abortivae and one to Corymbosae. The EDGE2
borderline and watch lists included 17 and 5 species respectively (Table A2 of Appendix). The watch list included widespread and locally abundant species
that belong to evolutionary distinctive sections such as S. lithosperma, S. melanomphala and S. tonkinensis.
Table 1. EDGE2 main list, i.e., threatened
species that scored above the median EDGE2 score of the entire genus at least
95% of the time. Species are ranked based on their EDGE2 score. IUCN RL: Red
List category of published species (RL IUCN: DD Data Deficient, VU Vulnerable,
EN Endangered, CR Critically Endangered), and preliminary assessment of Not
Evaluated species (i.e., Threatened). EDGE2: Evolutionarily Distinct and
Globally Endangered metric, ED2: evolutionary distinctiveness.
Tabla 1. Lista
principal EDGE2: especies amenazadas que obtuvieron una puntuación superior a
la mediana de la puntuación EDGE2 de todo el género al menos el 95% de las
veces. Las especies están ordenadas en función de su puntuación EDGE2. IUCN RL:
categoría de la Lista Roja de las especies publicadas (RL IUCN: DD Datos
Insuficientes, VU Vulnerable, EN En Peligro, CR En Peligro Crítico), y
evaluación preliminar de especies No Evaluadas. EDGE2: índice de Especies
Evolutivamente Distintas y Globalmente Amenazadas, ED2: distintividad evolutiva.
Scleria
species with the highest mean FUD scores were Scleria skutchii, the tallest species in the genus, and Scleria depressa, the species with the largest nutlet. Overall, 6 of 10 species with
the highest FUD scores belonged to the sections Ophryoscleria and Schizolepis
which include stout perennials with large leaf areas and climbers. The species
with the greatest mean EcoDGE score was Scleria porphyrocarpa, followed by Scleria tropicalis, Scleria williamsii and Scleria chlorantha. We found 38 EcoDGE species,
6 in the main list (Table 2) and 32 in the borderline EDGE2 species list (Table A3 of Appendix). These species (14.56% of species of the genus) account for 64.28%
of avertable expected functional diversity loss in the genus in a 50-year time
horizon.
The
evolutionary distinctiveness (ED2) metric was a poor explanatory variable of functional
distinctiveness (FUD) in Scleria (F1,237=0.03, p=0.86).
Table 2. EcoDGE main list, i.e., threatened
species that scored above the median EcoDGE score of the entire genus at least
95% of the time. Species are ranked based on their EcoDGE score. IUCN RL: Red
List category of published species (RL IUCN: CR Critically Endangered), and
preliminary assessment of Not Evaluated species (i.e., Threatened). EcoDGE:
Ecologically Distinct and Globally Endangered metric, FUD: functional
distinctiveness.
Tabla 2. Lista
principal de EcoDGE: especies amenazadas que obtuvieron una puntuación superior
a la mediana de la puntuación EcoDGE de todo el género al menos el 95% de las
veces. Las especies están ordenadas en función de su puntuación EcoDGE. IUCN
RL: categoría de la Lista Roja de las especies publicadas (RL IUCN: CR En
Peligro Crítico), y evaluación preliminar de especies No Evaluadas. EcoDGE:
Especies Ecológicamente Distintas y Globalmente Amenazadas, FUD: distintividad
funcional.
Regions of special interest for
conservation
The countries with the highest sum of EDGE2
scores and the greatest number of listed EDGE2 species were Madagascar,
Democratic Republic of the Congo, Brazil, Zambia and Tanzania (Table 3).
The ecoregions with the highest sum of EDGE2 scores and the greatest number of
listed EDGE2 species were the Central Zambezian Miombo woodlands, Madagascar
subhumid forests, Cerrado, East Sudanian savanna, Llanos, Guinean
forest-savanna mosaic and Madagascar lowland forests (Table 4; Fig. 2a).
The countries with the highest cumulative
EcoDGE scores were Brazil, Venezuela, Bolivia, Democratic Republic of the
Congo, Peru and Colombia. The countries with the greatest number of listed
EcoDGE species were Democratic Republic of the Congo, Brazil and Madagascar (Table 3).
The ecoregions with the highest sum of EcoDGE scores and number of listed
EcoDGE species were Central Zambezian Miombo woodlands, Southwest Amazon moist
forests, Bahia coastal forests, Cerrado and Western Congolian forest-savanna
mosaic (Table 4; Fig. 2b).
According to the assessment of the extent
of remaining natural habitat and protected land in the world ecoregions by Dinerstein et al. (2017), there are 22 Scleria
species which are restricted to ‘Nature Imperiled’ ecoregions (i.e., ecoregions
where the percentage of natural habitat remaining and the amount of the total
ecoregion that is protected is less than or equal to 20%) and 34 species only
occur in ecoregions where ‘Nature Could Recover (i.e., ecoregions where the
percentage of natural habitat remaining and area protected is less than 50% but
more than 20% and that require restoration programs to reach the 50% of natural
habitat).
Table 3. Top 20 countries in which Scleria
is present ranked by their sum of EDGE2 scores. Richness: number of species
present, N: number of EDGE2 and EcoDGE species, Main: number of species
included in the main EDGE2 and EcoDGE lists, Sum: sum of EDGE2 and EcoDGE
scores. Expanded results can be found in Table A4 of Appendix.
Tabla 3. Los 20
países con mayor riqueza de Scleria ordenados por la suma de sus
puntuaciones EDGE2. Riqueza: número de especies presentes, N: número de
especies EDGE2 y EcoDGE, Main: número de especies incluidas en las listas
principales EDGE2 y EcoDGE, Sum: suma de las puntuaciones EDGE2 y EcoDGE. Los resultados
ampliados se encuentran en la Tabla A4 del Apéndice 4.
Table 4. Top 20 ecoregions in which Scleria is present ranked by
their sum of EDGE2 scores. Richness: number of species present, N: number of
EDGE2 and EcoDGE species, Main: number of species included in the main EDGE2
and EcoDGE lists, Sum: sum of EDGE2 and EcoDGE scores. Expanded results
can be found in Table A5 of
Appendix.
Tabla 4. Las 20
principales ecorregiones en las que está presente Scleria clasificadas
por su suma de puntuaciones EDGE2. Riqueza: número de especies presentes, N:
número de especies EDGE2 y EcoDGE, Main: número de especies incluidas en las
listas principales EDGE2 y EcoDGE, Sum: suma de las puntuaciones EDGE2 y
EcoDGE. Los resultados ampliados se encuentran en la Tabla
A5 del Apéndice 5.
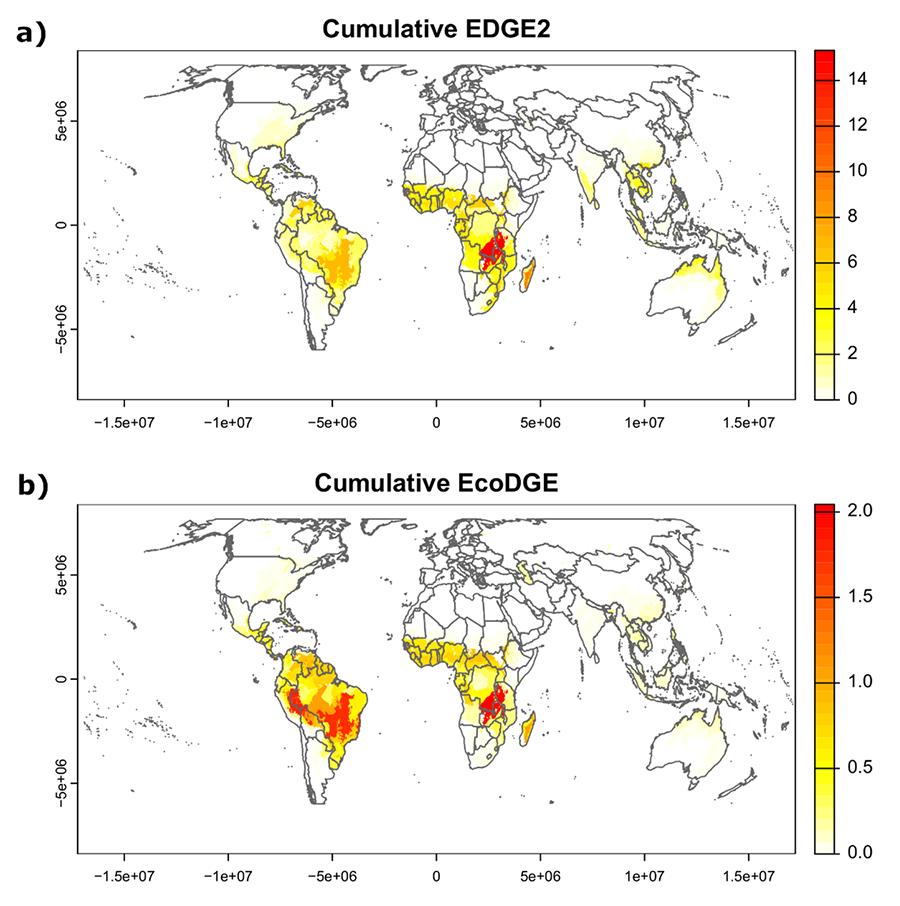
Figure 2. Ecoregions of notable conservation concern for Scleria according to
their sum of EDGE2 scores (a) and sum of EcoDGE scores (b). The definition of ecoregion follows Olson et al. (2001).
Figura 2. Ecorregiones de notable interés
para la conservación de Scleria según su suma de puntuaciones EDGE2 (a)
y suma de puntuaciones EcoDGE (b). La definición de
ecorregión se basa en Olson et
al. (2001).
Discussion
In this study, we estimated the extinction
risk for all species of the genus Scleria, as well as identified regions
of special interest for its conservation. Our results suggest that half of Scleria
species (49%, N=38) not yet included in the Red List are potentially threatened
with extinction. Evolutionary and ecologically distinct and endangered Scleria
mostly occur across African and South American regions.
Preliminary assessments
Similar to previous studies, RF
outperformed the method that explicitly followed Red List criterion B (Nic Lughadha et al. 2019) and distribution range properties were the
best predictors for RF (Nic Lughadha
et al. 2019; Bachman et al. 2023; Walker et al. 2023). Up to 15 species were classified as threatened by rCAT and
non-threatened by RF; thus, RF set lower thresholds of extent of occurrence
(EOO) to classify Scleria species as threatened. This seems a sensible
approach to assess Scleria because the size of the distribution range as
estimated from herbarium collections is potentially underestimated in most
cases (see below), and widespread and locally dominant species are common (Holm et al.
1979; Galán Díaz et al. 2019).
Considering species included in the Red
List and the results of RF, 26% of all Scleria species are potentially
threatened with extinction. In a recent study, Bachman et al. (2023) obtained a similar
estimate for Cyperaceae. They also found that, of the 21 families with more
than 3000 species, Cyperaceae showed the smallest proportion of threatened
species. Whereas 18% of Scleria species currently included in the Red
List are threatened, we found that 49% of unassessed species are potentially
threatened with extinction. This could be because several Madagascan and South
American Scleria species discovered over the last four decades were
classified as threatened by RF, which might support
that newly described species are geographically restricted and therefore less
likely to be encountered in the wild (Brown et al. 2023). This is the case of Scleria
nusbaumeri, Scleria
ankaratrensis, Scleria attenuatifolia, Scleria chasmema, Scleria millespicula, Scleria pernambucana, Scleria rubrostriata and Scleria tropicalis. It is therefore necessary to
prioritize the assessment of all unassessed species to get realistic estimates
of extinction risk in the genus and to galvanize conservation support for these
species.
Evolutionarily distinct and
globally endangered Scleria
Our analysis identified 23 species that met
the criteria to be included in the EDGE2 main list. Scleria porphyrocarpa
was the species with the highest EDGE2 and ED2 scores due to its high
vulnerability (i.e., it was classified as threatened by both rCAT and RF) and
high evolutionary distinctiveness. Scleria porphyrocarpa belongs to
section Corymbosae, the oldest lineage in subgenus Scleria (crown
age 22.28 Ma) which only includes four other species (Bauters et al.
2016, Larridon et al. 2021). Section Hypoporum was represented in the EDGE2 main list by
17 species. This is due to two reasons: (i) molecular analyses indicated that
section Hypoporum originated 8.4 Ma and showed a rapid increase in its
diversification rate soon after (Larridon et
al. 2021) to become the
richest section in Scleria (Bauters
et al. 2019); (ii) it
holds the highest percentage of threatened species among Scleria
sections according to the Red List and our preliminary assessments (42%; Fig. 1b).
This is important in the context of the new EDGE formulation, that accounts for
the extinction risk of closely related species, because the deeper branches of
a clade with a high proportion of threatened species will have a greater
probability of being at risk, thus resulting in species belonging to it having
higher EDGE scores (Gumbs et al.
2023). Finally, the three
threatened species of section Acriulus were also included in the main
list, as well as two Madagascan endemics from section Abortivae.
Almost half of Scleria
species (129 species) are present in the 11 ecoregions with the greatest
cumulative EDGE2 scores. Four African (Madagascar, D.R. Congo, Zambia and
Tanzania) and two South American (Brazil, Venezuela) countries mainly covered
these areas of special interest for conservation. Madagascar comprises 24
species of Scleria in two ecoregions (i.e., subhumid and lowland
forests), including five threatened and two recently described species.
Madagascan species belong to 11 sections out of the 17 sections recognized in Scleria,
which highlights the importance of this country as a reservoir of Scleria
diversity and the importance of long-distance dispersal in the genus (Galán Díaz et al. 2019). Four other
ecoregions from the Afrotropics (i.e., Central Zambezian Miombo woodlands, East
Sudanian Savanna, Guinean forest-savanna mosaic, and Northwestern Congolian
lowland forests and Zambezian and Mopane woodlands; Olson et al. 2001)
are identified as important conservation areas for Scleria. These
ecoregions contain 67 species (26% species of Scleria, from 12 sections)
of which 19 are endangered.
Brazil has the greatest
Scleria richness (63 species) and ranks second in terms of cumulative EDGE2
scores. Two Brazilian ecoregions are shown as areas of special interest for the
conservation of the evolutionary history of Scleria: Cerrado and Mato
Grosso seasonal forests. These ecoregions include 49 species from 11 sections,
of which 4 are endangered. The Venezuelan Llanos is another important ecoregion
for evolutionary distinct and endangered Scleria. Unlike the Colombian
Llanos, which also includes over 30 species, it includes species from old Scleria
lineages (i.e., sections Lithospermae and Foveolidia; Larridon et al. 2021)
and two threatened species.
According to the
revision of the world ecoregions of Dinerstein et al. (2017), which considered the World Database of Protected Areas (UNEP-WCMC y IUCN 2023) along with habitat assessments based on tree
cover and human land use, two of the above-mentioned ecoregions (i.e.,
Madagascar subhumid forests and Guinean forest-savanna mosaic) are classified
as ‘nature imperiled’ because the percentage of natural habitat remaining and protected area is less
than 20%. Other ecoregions of interest for Scleria conservation that
need active restoration programs to reach the 50% of protected natural habitat
are Madagascar lowland forests, Central Zambezian Miombo woodlands and East
Sudanian savanna.
Ecologically distinct and
globally endangered Scleria
We expect uncertainty
in the identification of EcoDGE species because the results are largely
dependent on the selection of traits, whereas the evolutionary relationship
among Scleria species is well resolved at least at
the section level (Bauters et al. 2016, 2018a). In this study we used traits which are
informative of species ecological strategies (Westoby 1998): plant longevity, leaf area, height and
nutlet size. These traits indicate how plants cope with environmental nutrient
stress and disturbances, the position of the species in the vertical light
gradient of the vegetation, competitive vigor, dispersal distance and seed
persistence in the soil bank (Pérez-Harguindeguy
et al. 2013). We found
ED2 and FUD were largely orthogonal; thus, EcoDGE based prioritizations yielded
complementary results to the EDGE2 approach.
Our analysis identified six species in the
main EcoDGE list. Unlike EDGE2, EcoDGE mostly points towards South American
countries as reservoirs of distinctive and endangered species. This is because
many EDGE2 species are phylogenetically restricted to section Hypoporum which
has its center of diversity in tropical Africa, whereas EcoDGE species (i.e.,
those with great blade area and nutlet size) belong to different sections
mostly restricted to South America. Eight South and Central American countries
are ranked among the top 10 countries with the highest cumulative EcoDGE
scores: Brazil, Venezuela, Bolivia, Peru, Colombia, Guyana, Dominican Republic
and Costa Rica. This is due to the occurrence of stout species from three
sections that are restricted or almost restricted to the Neotropics (Bauters
et al. 2016): sections
Schizolepsis (12 species), Ophryoscleria (11 species) and Hymenolytrum
(13 species). Within these countries, the ecoregions that showed the greatest
cumulative EcoDGE scores were Southwest Amazon moist forests, Bahia coastal
forests, Cerrado and Iquitos Varzeá (87 species in total). The percentage of
natural habitat remaining in these ecoregions is less than 50%, with
restoration programs needed to exceed this threshold (Dinerstein
et al. 2017). D. R.
Congo and Madagascar were the African countries with the highest cumulative
EcoDGE score.
Limitations
Preliminary assessments are biased
by the uncertainty and coverage of the taxonomic and geographical dimensions of
the occurrence datasets (Meyer et al.
2016). We expect little
uncertainty in the taxonomic dimension of our occurrence dataset because the
records have been manually curated and homogenized using the latest list of
accepted species names. Yet, there is a bias in the geographical coverage of Scleria
collections, where the available resources for each country vary greatly and
affect the density of observations as well as the number of specimens collected
and digitized. For instance, the United States of America and Australia are the
countries with the highest average number of Scleria observations per
species with 276 and 273 respectively, which is five times more than the third
and fourth countries in this ranking (Brazil and Mexico). However, the United
States of América and Australia rank 33rd and 11th in
terms of Scleria richness, whereas Brazil and México rank first and
thent. This affects the assessments of species from less explored regions by
potentially leading to an underestimation of the size of their distribution
ranges and a subsequent overestimation of their extinction risk (Nic Lughadha
et al. 2019). In
addition, the United States of America and Australia harbor two nearly endemic Scleria
subgenera (subgenera Trachylomia and Browniae, respectively).
Thus, the quality and quantity of Scleria collections do not only impact
the comprehensiveness of regional floras but it is also unevenly distributed
across the phylogeny. This uncertainty must be considered in biogeographical
studies.
There are also substantial differences in
the completeness of our knowledge of the genus Scleria among neighboring
countries. The identification of regions from Africa as areas of special
interest for the conservation of Scleria reflects their great diversity as
centers of diversification (Larridon et al. 2021), but also our more
comprehensive taxonomic knowledge of this group in particular countries. For
instance, Scleria from Zambia, South Africa and Madagascar are better studied
compared to other African countries thanks to the work of E.A. Robinson (Robinson 1966), E.F. Franklin Hennessy (Franklin Hennessy 1985) and H. Chermezon
(Chermezon 1937). Thus, remarkable patterns of
species richness in these countries might reflect different botanical
collection efforts rather than true species accumulation.
Finally, this study evaluates species and
ecosystems as individual elements, but modern biodiversity conservation
strategies will benefit from a thorough analysis of the eco-social landscape (Butler et al. 2022). On the one hand, the taxonomy of nut rushes is well-resolved (Bauters et al. 2016), but it still is a
little-studied genus in terms of its ecological and economic value. In a recent
bibliographical research, we found that nut rushes reported to have ecological
and economic values tend to be widespread (Galán Díaz et al. 2024), sometimes even
weeds that thrive in disturbed areas (Holm et al. 1979). Thus, we would
hypothesize that the conservation of these species will not be imperiled by
human activity. Still, the genus encompasses many narrow endemics and new
species are continuously discovered in poorly explored areas (Bauters et al. 2015; Mayedo y Thomas 2016; Bauters et al.
2018a; Bauters et al. 2018b; Galán Díaz et al. 2019; Schneider y
Gil 2021; Larridon et al. 2024). This geographical
bias in the coverage of Scleria collections previously commented on can be
tackled by collaborating with local researchers and communities in its
collection and taxonomic study (Heywood
2017). In regard, it is worth mentioning that
Scleria is mainly distributed across tropical areas but articles are often
published in subscription journals (for instance Lye y Pollard 2003; Strong 2007; Araújo y Brummitt 2011; Bauters et al.
2018b). This makes access to new findings
challenging for researchers from the Global South (Pettorelli et al. 2021). Thus, researchers from
the Global North have the responsibility to make papers and data freely
available worldwide for the sake of biodiversity conservation.
Conclusions
We provided the first global assessment of
species and world regions of remarkable interest for Scleria
conservation. We show that recent methodological advances in the identification
of species at-risk of extinction in combination with herbarium data allow the
implementation of the novel EDGE2 and EcoDGE frameworks in plant groups with
well-resolved taxonomies. We found that half of Scleria species not yet
included in the Red List are potentially at risk of extinction, and 21.5% of
the species in the genus are restricted to ecoregions where the percentage of
natural habitat is less than 50%. Phylogenetic and functional distinctiveness
metrics were largely uncorrelated and EDGE2 and EcoDGE metrics point toward
tropical regions of Africa and South America, respectively, as reservoirs of
distinctive and endangered species.
Data Accessibility
The data used to produce this study are
publicly available at Zenodo (Galán Díaz
et al. 2024).
The codes generated during the current
study are available at GitHub (https://github.com/galanzse/Scleria_EDGE).
Authors' contributions
JGD and IL originally formulated the idea.
JGD, HR and IL gathered and curated the data. JGD, SPB, FF and ME performed
statistical analyses. JGD, SPB, FF, ME, HR and IL wrote the manuscript. JGD and
IL acquired funding.
Funding, permissions required,
potential conflicts of interest and acknowledgements
This project was funded by the Spanish
Association of Terrestrial Ecology and ACOM LAB (Agrocomponentes). JGD
is supported by a Margarita Salas fellowship funded by the Spanish Ministry of
Universities and the European Union-Next Generation Plan (MSALAS-2022-22319).
The authors declare that they have no
conflict of interest.
We thank Martin Xanthos from the Royal
Botanic Gardens Kew, Germinal Rouhan and Thomas Haevermans from the Muséum
National d'Histoire Naturelle and the Spanish Ecological Association of
Terrestrial Ecology.
References
Araújo, A.C.,
Brummitt, N.A. 2011. Scleria rubrostriata, a
new species of Cyperaceae from the Brazilian Atlantic Forest. Kew Bulletin 66:
517-520. https://doi.org/10.1007/s12225-012-9321-4
Bachman,
S.P., Brown, M.J.M., Leão, T.C.C., Lughadha, E.N., Walker, B.E. 2023.
Extinction risk predictions for the world’s flowering plants to support their
conservation. bioRxiv. https://doi.org/10.1101/2023.08.29.555324
Bauters, K., Meganck, K., Vollesen, K., Goetghebeur, P., Larridon, I. 2015.
Scleria pantadenia and Scleria tricristata: Two new species of
Scleria subgenus hypoporum (Cyperaceae, Cyperoideae, Sclerieae) from
Tanzania. Phytotaxa 227: 45-54. https://doi.org/10.11646/phytotaxa.227.1.5
Bauters, K., Asselman, P., Simpson, D.A., Muasya, A.M., Goetghebeur, P.,
Larridon, I. 2016. Phylogenetics, ancestral state reconstruction, and a new
infrageneric classification of Scleria (Cyperaceae) based on three DNA markers.
Taxon 65: 444-466. https://doi.org/10.12705/653.2
Bauters, K., Goetghebeur, P., Asselman, P., Meganck, K., Larridon, I. 2018a.
Molecular phylogenetic study of Scleria subgenus Hypoporum (Sclerieae,
Cyperoideae, Cyperaceae) reveals several species new to science. https://doi.org/10.1371/journal.pone.0203478
Bauters, K., Goetghebeur, P., Larridon, I. 2018b. Scleria cheekii, a
new species of Scleria subgenus Hypoporum (Cyperaceae,
Cyperoideae, Sclerieae) from Cameroon. Kew Bulletin 73: 27. https://doi.org/10.1007/s12225-018-9752-7
Bauters, K.,
Larridon, I., Goetghebeur, P. 2019. A taxonomic study of scleria subgenus
hypoporum: Synonymy, typification and a new identification key. Phytotaxa 394: 1-49. https://doi.org/10.11646/phytotaxa.394.1.1
Booth, T.H., Nix, H.A., Busby, J.R., Hutchinson, M.F. 2014. bioclim: the
first species distribution modelling package, its early applications and
relevance to most current MaxEnt studies. Diversity and Distributions
20: 1-9. https://doi.org/10.1111/ddi.12144
Brown, M.J.M.M., Bachman, S.P., Nic Lughadha, E. 2023. Three in four
undescribed plant species are threatened with extinction. New Phytologist
240: 1340-1344. https://doi.org/10.1111/nph.19214
Butler, E.P., Bliss-Ketchum, L.L., de Rivera, C.E., Dissanayake, S.T.M.,
Hardy, C.L., Horn, D.A., Huffine, B., et al. 2022. Habitat, geophysical, and
eco-social connectivity: benefits of resilient socio–ecological landscapes. Landscape
Ecology 37: 1-29. https://doi.org/10.1007/s10980-021-01339-y
Chermezon, H. 1937. Cypéracées (29º Familie). En: Humbert, H. (ed.), Flore
de Madagascar et des Comores, Tananarive, Imprimerie officielle.
Antananarivo, Madagascar.
CIESIN 2016. Gridded Population of the World, Version 4 (GPWv4): Population
Density. Center for International Earth Science Information Network, Columbia
University. New York, USA. https://www.earthdata.nasa.gov/data/catalog/sedac-ciesin-sedac-gpwv4-popcount-r11-4.11
Cowell, C.R., Bullough, L.-A., Dhanda, S., Harrison Neves, V., Ikin, E.,
Moore, J., Purdon, R., et al. 2022. Fortuitous Alignment: The Royal
Botanic Gardens, Kew and the Sustainable Development Goals. Sustainability
14: 2366. https://doi.org/10.3390/su14042366
Davidse, G., Sánchez, M.S., Chater, A.O. 1994. Flora mesoamericana (Vol.
6). Alismataceae a Cyperaceae. UNAM, Missouri Botanical Garden and The
Natioanl History Museum, London, United Kingdom.
Dinerstein, E., Olson, D., Joshi, A., Vynne, C., Burgess, N.D., Wikramanayake,
E., Hahn, N., et al. 2017. An Ecoregion-Based Approach to Protecting Half
the Terrestrial Realm. BioScience 67: 534-545. https://doi.org/10.1093/biosci/bix014
Forest, F., Moat, J., Baloch, E., Brummitt, N.A., Bachman, S.P.,
Ickert-Bond, S., Hollingsworth, P.M., et al. 2018. Gymnosperms on the
EDGE. Scientific Reports 8: 1-11. https://doi.org/10.1038/s41598-018-24365-4
Franklin Hennessy, E.F. 1984. The genus Scleria
in southern Africa. Bothalia 530: 505-530. https://doi.org/10.4102/abc.v15i3/4.1829
Galán Díaz, J., Bauters,
K., Rabarivola, L., Xanthos, M., Goetghebeur, P., Larridon, I., Díaz, J.G.,
et al. 2019. A revision of scleria (Cyperaceae) in
Madagascar. Blumea: Journal of Plant Taxonomy and Plant Geography 64:
195-213.
Galán Díaz, J., Bauters,
K., Escudero, M., Larridon, I. 2024. Taxonomy, occurrences,
phylogeny, traits and uses of the entire plant genus Scleria (Cyperaceae) [Data set]. Available at: https://zenodo.org/records/12755860
GBIF 2023. GBIF Occurrence Download. Available at: https://doi.org/10.15468/dl.wvy3nc
[accessed 13/08/2023].
Govaerts, R., Nic Lughadha, E., Black, N., Turner, R., Paton, A. 2021. The
World Checklist of Vascular Plants, a continuously updated resource for
exploring global plant diversity. Scientific Data 8: 215. https://doi.org/10.1038/s41597-021-00997-6
Gower, J.C. 1971. A general coefficient of similarity and some of its properties. Biometrics
27: 857–871. https://doi.org/10.2307/2528823
Griffith, P., Lang, J.W., Turvey, S.T., Gumbs, R. 2022. Using functional
traits to identify conservation priorities for the world’s crocodylians. Functional
Ecology 1-13. https://doi.org/10.1111/1365-2435.14140
Gumbs, R., Gray, C.L., Wearn, O.R., Owen, N.R. 2018. Tetrapods on the
EDGE: Overcoming data limitations to identify phylogenetic conservation
priorities. PLOS ONE 13:
e0194680. https://doi.org/10.1371/journal.pone.0194680
Gumbs, R., Gray, C.L., Böhm, M., Burfield, I.J., Couchman, O.R., Faith,
D.P., Forest, F., et al. 2023. The EDGE2 protocol: Advancing the
prioritisation of Evolutionarily Distinct and Globally Endangered species for
practical conservation action. PLoS Biology 21: 1-22. https://doi.org/10.1371/journal.pbio.3001991
Heywood, V.H. 2017. Plant conservation in the Anthropocene – Challenges and
future prospects. Plant Diversity 39: 314-330. https://doi.org/10.1016/j.pld.2017.10.004
Hidasi-Neto, J., Loyola, R., Cianciaruso, M.V. 2015. Global
and local evolutionary and ecological distinctiveness of terrestrial mammals:
identifying priorities across scales. Diversity and Distributions 21:
548-559. https://doi.org/10.1111/ddi.12320
Holm, L., Pancho, J.V., Herberger, J.P., Plucknett, D.L. 1979. A
geographical atlas of world weeds. John Wiley and Sons. New York, USA.
Huang, D. 2012. Threatened Reef Corals of the World Matz, M.V. (ed.),. PLoS ONE
7: e34459. https://doi.org/10.1371/journal.pone.0034459
iNaturalist community. Research grade observations of
all Scleria species worldwide available on 23/10/2023. [Exported from https://www.inaturalist.org on 23/10/2023].
Isaac, N.J.B., Turvey, S.T., Collen, B., Waterman, C., Baillie, J.E.M.
2007. Mammals on the EDGE: Conservation priorities based on threat and
phylogeny. PLoS ONE 2(3):e296. https://doi.org/10.1371/journal.pone.0000296
IUCN 2023. The IUCN Red List of Threatened Species. Version 2023-1 [accessed 13/08/2023].
Kondratyeva, A., Grandcolas, P., Pavoine, S. 2019. Reconciling the concepts and
measures of diversity, rarity and originality in ecology and evolution. Biological
Reviews 94: 1317-1337. https://doi.org/10.1111/brv.12504
Larridon, I. 2022. A linear classification of Cyperaceae. Kew Bulletin 77:
309-315. https://doi.org/10.1007/s12225-022-10010-x
Larridon, I., Galán Díaz,
J., Bauters, K., Escudero, M. 2021. What drives
diversification in a pantropical plant lineage with extraordinary capacity for
long-distance dispersal and colonization? Journal of Biogeography 48:
64-77. https://doi.org/10.1111/jbi.13982
Larridon, I., Bauters, K., Rasaminirina, F., Galán Díaz, J., Márquez-Corro,
J.I., Gautier, L. 2024. A new remarkable species of Scleria
(Cyperaceae) from northern Madagascar. Candollea 79: 107-116. https://doi.org/10.15553/c2024v791a6
Le Roux, M. 2015. The e-Flora of South Africa. Veld & Flora
101: 12.
Losos, J.B. 2008. Phylogenetic niche conservatism, phylogenetic signal and the
relationship between phylogenetic relatedness and ecological similarity among
species. Ecology Letters 11: 995-1003. https://doi.org/10.1111/j.1461-0248.2008.01229.x
Lye, K.A., Pollard, B.J. 2003. Studies in African Cyperaceae 29. Scleria
afroreflexa, a new species from western Cameroon. Nordic Journal of Botany
23: 431-435. https://doi.org/10.1111/j.1756-1051.2003.tb00416.x
Mayedo, W.B., Thomas, W.W. 2016. Two New Species of Scleria section
Hypoporum (Cyperaceae) from Espírito Santo, Brazil. Phytotaxa 268: 263. https://doi.org/10.11646/phytotaxa.268.4.4.
Meyer, C., Weigelt, P., Kreft, H. 2016. Multidimensional biases, gaps and
uncertainties in global plant occurrence information Lambers, J. H. R. (ed.),. Ecology
letters 19: 992-1006. ttps://doi.org/10.1111/ele.12624
Moat, J. Bachman, S. 2020. rCAT: Conservation
assessment tools. R package version 0.1.6. https://CRAN.R-project.org/package=rCAT
Mooers, A.Ø., Faith, D.P., Maddison, W.P. 2008. Converting Endangered
Species Categories to Probabilities of Extinction for Phylogenetic Conservation
Prioritization. PLoS ONE 3: e3700. https://doi.org/10.1371/journal.pone.0003700
Mu, H., Li, X., Wen, Y., Huang,
J., Du, P., Su, W., Miao, S., et al. 2022. A global record of
annual terrestrial Human Footprint dataset from 2000 to 2018. Scientific
Data 9:176. https://doi.org/10.1038/s41597-022-01284-8
Nic
Lughadha, E., Walker, B.E., Canteiro, C., Chadburn,
H., Davis, A.P., Hargreaves, S., Lucas, E.J., et al. 2019. The use and
misuse of herbarium specimens in evaluating plant extinction risks. Philosophical
Transactions of the Royal Society B: Biological Sciences 374: 20170402. https://doi.org/10.1098/rstb.2017.0402
Olson, D.M.,
Dinerstein, E., Wikramanayake, E.D., Burgess, N.D., Powell, G.V.N., Underwood,
E.C., D’Amico, J.A. et al. 2001. Terrestrial ecoregions of the world: a
new map of life on Earth. Bioscience 51: 933-938. https://doi.org/10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2
Pérez-Harguindeguy, N., Diaz, S., Garnier, E., Lavorel, S., Poorter, H., Jaureguiberry,
P., Bret-Harte, M.S.S., et al. 2013. New Handbook for standardized
measurement of plant functional traits worldwide. Australian Journal of
Botany 61: 167-234. https://doi.org/10.1071/BT12225
Pettorelli, N., Barlow, J., Nuñez, M.A., Rader, R., Stephens, P.A., Pinfield,
T., Newton, E. 2021. How international journals can support ecology from the
Global South. Journal of Applied Ecology 58: 4-8. https://doi.org/10.1111/1365-2664.13815
Ramos-Gutiérrez, I., Lima, H., Vilela, B., Molina-Venegas, R. 2023. A generalized framework to expand incomplete phylogenies using non-molecular phylogenetic information. Global Ecology and Biogeography 1-10.
https://doi.org/10.1111/geb.13733
Robinson, E. 1966. An Account of the Genus Scleria in the Flora Zambesiaca. Kew
Bulletin 18: 487-551. https://doi.org/10.2307/4115799
Safi, K., Armour-Marshall, K., Baillie, J.E.M., Isaac, N.J.B. 2013.
Global Patterns of Evolutionary Distinct and Globally Endangered Amphibians and
Mammals. PLoS ONE 8: e63582. https://doi.org/10.1371/journal.pone.0063582
Šavrič, B., Patterson, T., Jenny, B. 2019. The Equal Earth map projection.
International Journal of Geographical Information Science 33: 454-465. https://doi.org/10.1080/13658816.2018.1504949
Schneider, L.J.C., Gil, A.D.S.B. 2021. Scleria (Cyperaceae) in the
state of Pará, Amazon, Brazil. Acta Botanica Brasilica 35: 215-247. https://doi.org/10.1590/0102-33062020abb0221
Simpson, D.A.,
Inglis, C.A. 2001. Cyperaceae of economic,
ethnobotanical and horticultural importance: a checklist. Kew Bulletin 56: 257
–360.
Simpson,
D.A., Koyama, T. 1998. Flora of Thailand. Vol.
6, part 4, Cyperaceae. Forest Herbarium, Rotal Forest Department, Bangkok.
Thailand.
Smith, J.R., Letten, A.D., Ke, P.-J.J., Anderson, C.B., Hendershot, J.N.,
Dhami, M.K., Dlott, G.A., et al. 2018. A global test of ecoregions. Nature
Ecology and Evolution 2: 1889-1896. https://doi.org/10.1038/s41559-018-0709-x
Strong, M.T. 2007. Scleria tropicalis (Cyperaceae), A new species
from Northern Andean South America. Harvard Papers in Botany 11:
199-201. https://doi.org/10.3100/1043-4534(2007)11[199:STCANS]2.0.CO;2
UNEP-WCMC,
IUCN. 2023. Protected Planet: The World Database
on Protected Areas (WDPA) and World Database on Other Effective Area-based
Conservation Measures (WD-OECM), [08/2023]. UNEP-WCMC
and IUCN, Cambridge, UK. Availaible at: https://www.protectedplanet.net.
Walker, B.E., Leão, T.C.C., Bachman, S.P., Lucas, E., Nic Lughadha, E.
2023. Evidence-based guidelines for automated conservation assessments of plant
species. Conservation Biology 37: 1-12. https://doi.org/10.1111/cobi.13992
Westoby, M.
1998. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant and Soil
199: 213-227. https://doi.org/10.1023/A:1004327224729
Zizka, A., Andermann, T., Silvestro, D. 2022. IUCNN – Deep learning
approaches to approximate species’ extinction risk. Diversity and
Distributions 28: 227-241. https://doi.org/10.1111/ddi.13450
Appendix
Table A1. Combined Red List results. This
includes assessments published in the Red List of the IUCN version 2023-1 (RL
IUCN: NE Not Evaluated, DD Data Deficient, LC Least Concern, NT Near
Threatened, VU Vulnerable, EN Endangered, CR Critically Endangered, EX
Extinct), unpublished assessments (‘Assessed but not published’) and
preliminary assessments (T Threatened, NT Non-threatened) of 67 species for
which occurrence data was available. Preliminary assessments followed two
approaches.
(i) Random forest approach (RF). We used as predictors: EOO, AOO, mean of latitudinal range,
elevation, minimum human population density in 2020 (HPD; Center for International Earth Science Information Network - CIESIN 2016), human footprint index in 2013 (HFI; Mu et al. 2022), proportion of observations located in
protected areas (UNEP-WCMC
and IUCN 2023), annual mean
temperature, minimum temperature of the coldest month, temperature annual
range, annual precipitation, precipitation of the driest month and
precipitation seasonality. We performed 10 repeats of 5-fold cross-validation
to train and evaluate the model and retained 20% of data for external
validation.
(ii)
rCAT. We implemented the function ‘ConBatch’ from
the R package ‘rCAT’ (Moat & Bachman
2020) to assess species’
extinction risk following IUCN Criterion B based on EOO.
Tabla A1. Resultados combinados de riesgo de
extinción. Esto incluye evaluaciones publicadas en la Lista Roja de la UICN
versión 2023-1 (RL UICN: NE No evaluado, DD Datos insuficientes, LC
Preocupación menor, NT Casi amenazado, VU Vulnerable, EN En peligro, CR En
peligro crítico, EX Extinto), evaluaciones no publicadas (‘Assessed but not
published’) y evaluaciones preliminares (Amenazado: T, No amenazado: NT) de 67
especies para las que se disponía de datos de presencia. Las evaluaciones
preliminares siguieron dos enfoques.
(i) Enfoque de bosque aleatorio (RF). Se utilizaron
como predictores EOO, AOO, media del rango latitudinal, elevación, densidad
mínima de población humana en 2020 (HPD; Center for International Earth Science
Information Network - CIESIN 2016), índice de huella
humana en 2013 (HFI; Mu et al. 2022), proporción
de observaciones localizadas en áreas protegidas (UNEP-WCMC
and IUCN 2023), temperatura media anual, temperatura mínima del mes más
frío, rango anual de temperatura, precipitación anual, precipitación del mes
más seco y estacionalidad de la precipitación. Realizamos 10 repeticiones de
validación cruzada con 5 particiones para entrenar y evaluar el modelo y
retuvimos el 20% de los datos para la validación externa.
(ii) rCAT. Implementamos la función 'ConBatch' del
paquete R 'rCAT' (Moat & Bachman 2020) para
evaluar el riesgo de extinción de las especies siguiendo el Criterio B de la
UICN basado en EOO.
Species
without occurrence data / Especies sin datos de presencia: S. assamica (C.B.Clarke) D.M.Verma, S.
depauperata Boeckeler, S. elongatissima Piérart, S. hirta
Boeckeler, S. jiangchengensis Y.Y.Qian, S. lateritica Nelmes, S.
macrolomioides H.Pfeiff., S. mutoensis Nakai, S. papuana
J.Kern, S. scandens Core, S. schenckiana Boeckeler and S.
swamyi Govind.
Table A2. Scleria EDGE2 borderline and watch lists
(Gumbs et al. 2023). Borderline list: threatened
species whose distribution of EDGE2 scores rank above the median of the entire
genus at least 80% of the time. Watch list: nonthreatened species whose distribution of EDGE2 scores rank above
the median of the entire genus at least 95% of the times. EDGE2: Evolutionarily Distinct and
Globally Endangered metric, ED2: evolutionary distinctiveness.
Tabla A2. Listas límite y de vigilancia EDGE2
de Scleria (Gumbs et al. 2023). Lista
límite: especies amenazadas cuya distribución de las puntuaciones EDGE2 se
sitúa por encima de la mediana de todo el género al menos el 80% de las veces.
Lista de vigilancia: especies no amenazadas cuya distribución de las
puntuaciones EDGE2 se sitúa por encima de la mediana de todo el género al menos
el 95% de las veces. EDGE2: Métrica de distintividad evolutiva y en peligro de
extinción a escala mundial, ED2: Métrica de distintividad evolutiva.
Table A3. Scleria EcoDGE borderline list (Griffith et al.
2022; Gumbs et al. 2023): threatened species whose distribution of EcoDGE scores rank above
the median of the entire genus at least 80% of the time. EcoDGE: Ecologically
Distinct and Globally Endangered metric, FUD: functional distinctiveness.
Tabla A3. Lista límite EcoDGE de Scleria
(Griffith et al. 2022; Gumbs
et al. 2023): especies amenazadas cuya distribución de puntuaciones EcoDGE
se sitúa por encima de la mediana de todo el género al menos el 80% de las
veces. EcoDGE: Métrica de distintividad ecológica y en peligro de extinción a
escala mundial, FUD: distintividad funcional.
Table A4. Countries in which Scleria is present ranked by their sum
of EDGE2 scores. Richness: number of species present, N: number of listed EDGE2
and EcoDGE species, Main: number of species included in the main EDGE2 and
EcoDGE lists, Sum: sum of EDGE2 and EcoDGE scores.
Tabla A4. Países en los que Scleria
está presente ordenados por su suma de puntuaciones EDGE2. Richness: número de
especies presentes, N: número de especies incluidas en las listas EDGE2 y
EcoDGE, Main: número de especies incluidas en las listas principales EDGE2 y
EcoDGE, Sum: suma de las puntuaciones EDGE2 y EcoDGE.
|
Country
|
Richness
|
EDGE2
|
EcoDGE
|
|
N
|
Main
|
Sum
|
N
|
Main
|
Sum
|
|
Madagascar
|
24
|
7
|
3
|
10.11
|
3
|
0
|
1.24
|
|
Dem. Rep. Congo
|
26
|
5
|
2
|
9.85
|
5
|
0
|
1.94
|
|
Brazil
|
63
|
6
|
2
|
9.43
|
4
|
2
|
3.08
|
|
Zambia
|
29
|
5
|
4
|
8.95
|
1
|
0
|
0.79
|
|
Tanzania
|
30
|
5
|
3
|
8.23
|
2
|
0
|
0.63
|
|
Venezuela
|
31
|
3
|
1
|
5.94
|
1
|
1
|
2.00
|
|
South Africa
|
17
|
2
|
0
|
5.23
|
0
|
0
|
0.17
|
|
Thailand
|
20
|
3
|
1
|
5.18
|
0
|
0
|
0.28
|
|
China
|
15
|
3
|
0
|
4.76
|
0
|
0
|
0.24
|
|
Bolivia
|
34
|
1
|
0
|
4.33
|
1
|
0
|
1.98
|
|
Guinea
|
15
|
4
|
2
|
4.29
|
1
|
0
|
0.22
|
|
Ghana
|
20
|
2
|
0
|
4.20
|
0
|
0
|
0.68
|
|
Ethiopia
|
16
|
4
|
1
|
4.20
|
0
|
0
|
0.29
|
|
Côte d'Ivoire
|
20
|
2
|
0
|
4.05
|
0
|
0
|
0.66
|
|
Philippines
|
13
|
3
|
0
|
3.96
|
1
|
0
|
0.32
|
|
Gabon
|
19
|
3
|
1
|
3.95
|
2
|
0
|
0.66
|
|
Angola
|
11
|
2
|
1
|
3.83
|
1
|
0
|
0.32
|
|
Nigeria
|
20
|
2
|
0
|
3.74
|
0
|
0
|
0.63
|
|
Guyana
|
21
|
1
|
0
|
3.58
|
1
|
0
|
1.26
|
|
Cameroon
|
18
|
3
|
0
|
3.56
|
2
|
0
|
0.89
|
|
Australia
|
22
|
1
|
0
|
3.51
|
1
|
0
|
0.30
|
|
Zimbabwe
|
8
|
2
|
1
|
3.50
|
1
|
0
|
0.21
|
|
Colombia
|
32
|
1
|
0
|
3.47
|
0
|
0
|
1.41
|
|
Mozambique
|
7
|
2
|
0
|
3.45
|
0
|
0
|
0.23
|
|
India
|
13
|
2
|
0
|
3.45
|
2
|
0
|
0.51
|
|
Mexico
|
23
|
1
|
0
|
3.39
|
0
|
0
|
0.54
|
|
Cuba
|
22
|
1
|
0
|
3.26
|
0
|
0
|
0.36
|
|
Indonesia
|
15
|
1
|
0
|
3.14
|
1
|
1
|
0.40
|
|
Belize
|
16
|
1
|
0
|
3.03
|
0
|
0
|
0.42
|
|
eSwatini
|
6
|
2
|
0
|
2.94
|
0
|
0
|
0.07
|
|
Guatemala
|
13
|
1
|
0
|
2.83
|
0
|
0
|
0.44
|
|
Nicaragua
|
15
|
1
|
0
|
2.82
|
0
|
0
|
0.45
|
|
Honduras
|
16
|
1
|
0
|
2.80
|
0
|
0
|
0.46
|
|
Costa Rica
|
17
|
1
|
0
|
2.70
|
0
|
0
|
0.98
|
|
Trinidad and Tobago
|
13
|
1
|
0
|
2.62
|
1
|
1
|
0.74
|
|
Liberia
|
10
|
2
|
1
|
2.58
|
0
|
0
|
0.18
|
|
Burkina Faso
|
11
|
1
|
0
|
2.51
|
0
|
0
|
0.53
|
|
Benin
|
18
|
1
|
0
|
2.50
|
0
|
0
|
0.61
|
|
Peru
|
24
|
1
|
0
|
2.45
|
1
|
0
|
1.53
|
|
Eq. Guinea
|
9
|
2
|
1
|
2.42
|
1
|
0
|
0.31
|
|
Uganda
|
11
|
1
|
0
|
2.36
|
0
|
0
|
0.27
|
|
Botswana
|
8
|
1
|
0
|
2.27
|
0
|
0
|
0.08
|
|
Togo
|
12
|
1
|
0
|
2.16
|
0
|
0
|
0.56
|
|
Dominican Rep.
|
8
|
2
|
0
|
2.08
|
1
|
0
|
1.01
|
|
United States of America
|
14
|
1
|
0
|
2.06
|
0
|
0
|
0.12
|
|
Malawi
|
6
|
1
|
1
|
2.01
|
0
|
0
|
0.21
|
|
Taiwan
|
8
|
1
|
0
|
1.95
|
0
|
0
|
0.09
|
|
Sri Lanka
|
6
|
1
|
0
|
1.84
|
0
|
0
|
0.04
|
|
Argentina
|
12
|
1
|
0
|
1.83
|
0
|
0
|
0.39
|
|
Puerto Rico
|
11
|
1
|
0
|
1.77
|
0
|
0
|
0.25
|
|
Sierra Leone
|
8
|
1
|
1
|
1.68
|
0
|
0
|
0.10
|
|
Panama
|
15
|
0
|
0
|
1.65
|
1
|
0
|
0.96
|
|
Congo
|
11
|
1
|
0
|
1.64
|
1
|
0
|
0.33
|
|
Jamaica
|
6
|
1
|
0
|
1.55
|
0
|
0
|
0.16
|
|
Kenya
|
5
|
1
|
0
|
1.48
|
0
|
0
|
0.17
|
|
Paraguay
|
12
|
0
|
0
|
1.47
|
0
|
0
|
0.40
|
|
Central African Rep.
|
10
|
0
|
0
|
1.31
|
0
|
0
|
0.16
|
|
Fiji
|
2
|
1
|
0
|
1.29
|
0
|
0
|
0.01
|
|
Tonga
|
2
|
1
|
0
|
1.29
|
0
|
0
|
0.01
|
|
Suriname
|
17
|
0
|
0
|
1.25
|
0
|
0
|
0.70
|
|
Rwanda
|
2
|
1
|
0
|
1.24
|
0
|
0
|
0.02
|
|
Bahamas
|
1
|
1
|
0
|
1.22
|
0
|
0
|
0.01
|
|
Timor-Leste
|
1
|
1
|
0
|
1.22
|
0
|
0
|
0.01
|
|
Cayman Is.
|
1
|
1
|
0
|
1.22
|
0
|
0
|
0.01
|
|
Ecuador
|
16
|
0
|
0
|
1.17
|
0
|
0
|
0.47
|
|
Guinea-Bissau
|
5
|
0
|
0
|
1.00
|
0
|
0
|
0.09
|
|
Niger
|
2
|
0
|
0
|
0.76
|
0
|
0
|
0.03
|
|
S. Sudan
|
1
|
1
|
0
|
0.74
|
1
|
0
|
0.10
|
|
New Caledonia
|
4
|
1
|
0
|
0.72
|
2
|
1
|
0.28
|
|
Chad
|
4
|
0
|
0
|
0.50
|
0
|
0
|
0.15
|
|
Japan
|
6
|
0
|
0
|
0.49
|
0
|
0
|
0.07
|
|
Mali
|
7
|
0
|
0
|
0.48
|
0
|
0
|
0.47
|
|
Lesotho
|
3
|
0
|
0
|
0.43
|
0
|
0
|
0.01
|
|
Senegal
|
9
|
0
|
0
|
0.41
|
0
|
0
|
0.50
|
|
Nepal
|
3
|
0
|
0
|
0.41
|
0
|
0
|
0.03
|
|
Burundi
|
5
|
0
|
0
|
0.40
|
0
|
0
|
0.16
|
|
Myanmar
|
2
|
0
|
0
|
0.40
|
0
|
0
|
0.02
|
|
El Salvador
|
5
|
0
|
0
|
0.34
|
0
|
0
|
0.06
|
|
Vietnam
|
5
|
0
|
0
|
0.32
|
0
|
0
|
0.07
|
|
Papua New Guinea
|
3
|
0
|
0
|
0.25
|
0
|
0
|
0.02
|
|
Cambodia
|
3
|
0
|
0
|
0.23
|
1
|
0
|
0.08
|
|
Seychelles
|
2
|
0
|
0
|
0.19
|
0
|
0
|
0.04
|
|
Mauritius
|
1
|
0
|
0
|
0.19
|
1
|
0
|
0.06
|
|
Vanuatu
|
2
|
0
|
0
|
0.15
|
0
|
0
|
0.01
|
|
Laos
|
2
|
0
|
0
|
0.09
|
0
|
0
|
0.02
|
|
Uruguay
|
1
|
0
|
0
|
0.08
|
0
|
0
|
0.00
|
|
Malaysia
|
1
|
0
|
0
|
0.02
|
0
|
0
|
0.01
|
|
French Guiana
|
0
|
0
|
0
|
0.00
|
0
|
0
|
0.00
|
Table A5. Top
50 ecoregions in which Scleria is present ranked by their sum of EDGE2
scores. Richness: number of species present, N: number of EDGE2 and EcoDGE
species, Main: number of species included in the main EDGE2 and EcoDGE lists,
Sum: sum of EDGE2 and EcoDGE scores.
Tabla A5. Las 50 principales ecorregiones en
las que Scleria está presente según su suma de puntuaciones EDGE2. Richness:
número de especies presentes, N: número de especies EDGE2 y EcoDGE, Main:
número de especies incluidas en las listas principales EDGE2 y EcoDGE, Sum:
suma de las puntuaciones EDGE2 y EcoDGE.
![]() , Steven P.
Bachman3
, Steven P.
Bachman3 ![]() , Félix Forest3
, Félix Forest3 ![]() , Marcial Escudero1
, Marcial Escudero1 ![]() , Hannah Rotton3, Isabel Larridon3,4
, Hannah Rotton3, Isabel Larridon3,4 ![]()

